What are valence electrons and why it’s important?
The total number of electrons in the last shell of an atom is called the valence electrons. That is, the total number of electrons in the last orbit of an element after electron configuration is called the valence electron.
The valence electrons of the element play an important role in that element. It participates in the formation of bonds and compounds of one element with another.
This article discusses in detail how to easily determine the number of valence electrons in an element. Hopefully, after reading this article you will know the details about this topic.
What are valence electrons?
The nucleus is located at the center of the atom. Protons and neutrons are located in the nucleus. The electrons are located around the nucleus at a certain distance and in a certain circular path.
These circular pathways are called shell or energy levels. This shell or energy level is known as orbit. According to scientist Niels Bohr, energy levels are three-dimensional spherical. That is, the shells are somewhat like football-shaped spheres.
The shell closest to the nucleus is the first orbit. This first shell is the smallest. This shell can hold a maximum of two electrons. The second shell is larger than the first shell. This orbit(shell) can have a maximum of eight electrons.
As the shell gets bigger, the electron holding capacity in the shell increases. The total number of electrons in the last shell after arranging the electrons of the element is called the valence electron. Depending on the element, the valence electrons may be paired or unpaired.
Where are the valence electrons located in the atom?
We already know what the valence electron is about. This time we will know where the valence electron is located in the atom. To know the details about the valence electrons first you have to know about the electron configuration of the element.
However, the answer to the question of where the valence electron is located in the atom is that the valence electron is located in the last orbit of the atom.
That is, the total number of electrons in the last orbit of an atom is the number of valence electrons in that element. However, in order to determine the number of valence electrons of a particular atom, one must know the electron configuration of that atom.
How to find valence electrons in an atom?
The electrons in the last orbit of the atom are the valence electrons. The valence electrons are in the last orbit of the atom. To determine the number of valence electrons of a particular atom, the electrons of that element have to be arranged.
However, in addition to the electron configuration, valence electrons can be determined by group and block in the periodic table.
Determination of valence electrons by electron configuration
The simplest and most accurate way to determine the valence electron is to know in detail the electron configuration of that element. Valence electrons can not be accurately determined by block and group.
But valence electrons can be easily identified by electron configuration. This time we will see how to arrange the electrons of an element. Many rules have to be followed to arrange the electrons of the element.
For example Aufbau principle, Hund’s principle, and Pauli’s exclusion principle. However, it is possible to easily determine the valence electron if the Bohr principal knows.
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there.
The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit]
K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2.

For example,
- n = 1 for K orbit.
The electron holding capacity of K orbit is 2n2 = 2 × 12 = 2 electrons. - For L orbit, n = 2.
The electron holding capacity of the L orbit is 2n2 = 2 × 22 = 8 electrons. - n=3 for M orbit.
The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. - n=4 for N orbit.
The maximum electron holding capacity in N orbit is 2n2 = 2 × 42 = 32 electrons.
Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons.
We already know that the total number of electrons in the last orbit of an atom is the number of valence electrons in that element. For example, the atomic number of oxygen is eight. Therefore, the atom of the oxygen element has a total of eight electrons.
Therefore, an oxygen atom will have two electrons in the first shell and six in the 2nd shell. Therefore, the order of the number of electrons in each shell of the oxygen(O) atom is 2, 6.
From the electron configuration of oxygen atoms, we see that the second orbit of oxygen is the last shell and there are a total of six electrons in the last orbit. So, we can easily say that the oxygen atom has six valence electrons.
Similarly, the atomic number of sulfur is 16. Therefore, the total number of electrons in a sulfur atom is 16. The electron configuration of sulfur shows that there are a total of two electrons in the first orbit and a total of eight electrons in the second orbit.
And the third orbit has a total of six electrons. From the electron configuration of sulfur, it is understood that the third orbit is the last orbit of sulfur and there are a total of six electrons. So, we can easily say that the number of valence electrons of sulfur is six.
Determination of valence electrons through the electron configuration of the Aufbau principle
Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub-energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, f. Determining the value of ‘l’ for different energy levels is-
- If n = 1,
(n – 1) = (1–1) = 0
Therefore, the orbital number of ‘l’ is 1; And the orbital is 1s. - If n = 2,
(n – 1) = (2–1) = 1.
Therefore, the orbital number of ‘l’ is 2; And the orbital is 2s, 2p. - If n = 3,
(n – 1) = (3–1) = 2.
Therefore, the orbital number of ‘l’ is 3; And the orbital is 3s, 3p, 3d. - If n = 4,
(n – 1) = (4–1) = 3
Therefore, the orbital number of ‘l’ is 4; And the orbital is 4s, 4p, 4d, 4f. - If n = 5,
(n – 1) = (n – 5) = 4.
Therefore, l = 0,1,2,3,4. The number of orbitals will be 5 but 4s, 4p, 4d, 4f in these four orbitals it is possible to arrange the electrons of all the elements of the periodic table.
The electron holding capacity of these orbitals is s = 2, p = 6, d = 10 and f = 14. The German physicist Aufbau first proposed the idea of electron configuration through sub-orbits.

The Aufbau method is to do electron configuration through the sub-energy level. The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital.
These orbitals are named s, p, d, f. The Aufbau electron configuration method is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d.
The atomic number of nitrogen is seven. Therefore, the total number of electrons in a nitrogen atom is seven. The electron configuration of nitrogen shows that there are two electrons in the first orbit and a total of five electrons in the second orbit.
From the electron configuration of nitrogen, it is understood that the second orbit of nitrogen is the last orbit. The electron configuration of nitrogen shows that there are two electrons in the first orbit and a total of five electrons in the second orbit.
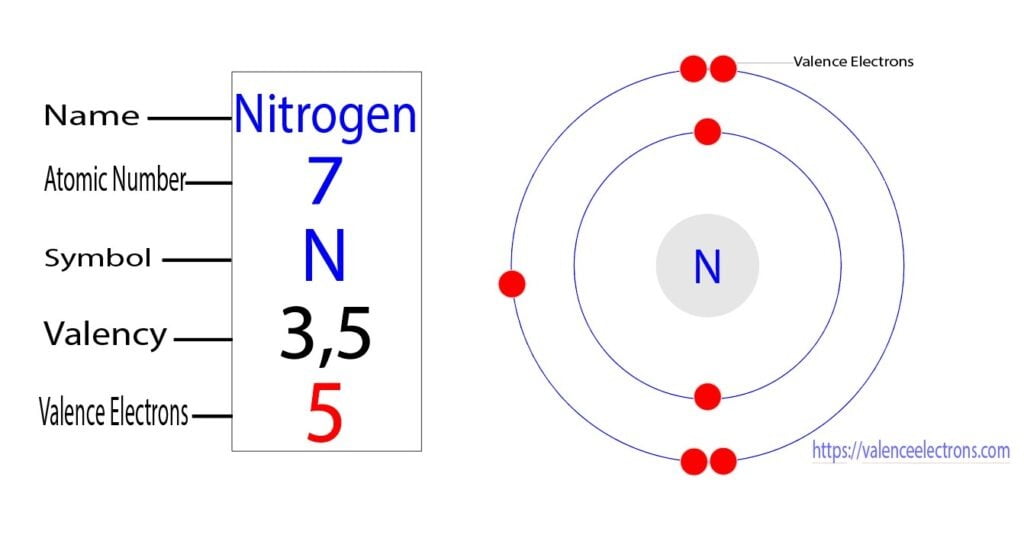
The electron configuration indicates that the second orbit of nitrogen is the last orbit and the last orbit has a total of five electrons. Therefore, we can easily say that the nitrogen atom has a total of five valence electrons.
Similarly, the atomic number of argon is 18. The total number of electrons in an argon atom is 18. The electron configuration of argon shows that there are two electrons in the first shell, eight in the 2nd orbit, and eight electrons in the 3rd shell.
From the electron configuration of argon, we understand that the third orbit of argon is the last orbit and the last shell has a total of eight electrons. Therefore, the valence electrons in argon are eight.
Determination of valence electrons by group
Valence electrons can be easily identified by groups and blocks. However, this method has several limitations in determining valence electrons. There are a total of 18 groups in the periodic table.
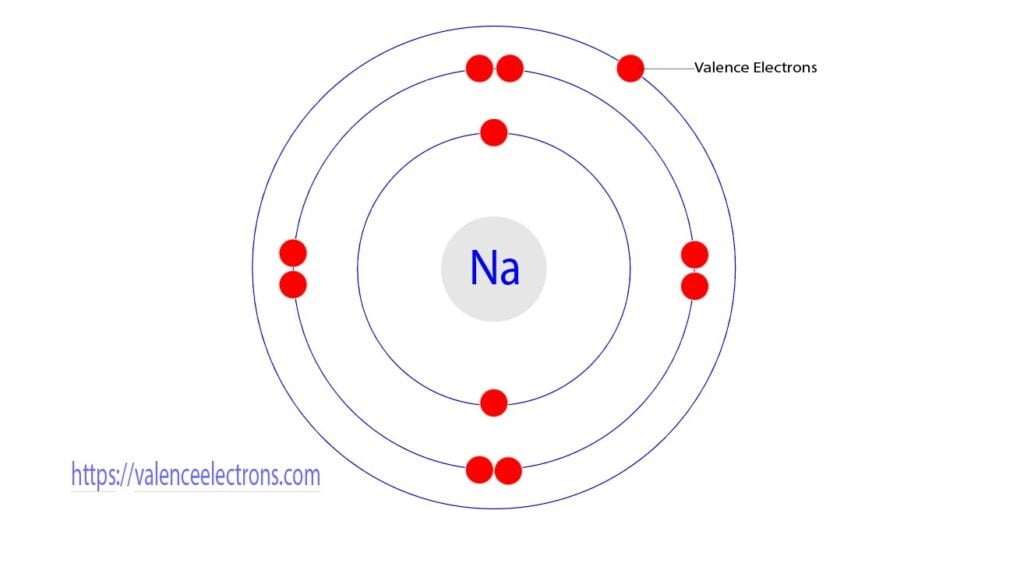
Group-1 elements are called alkali metals. The number of valence electrons in all the elements in this group is one. For example, the electron configuration of sodium shows that sodium is an element of group-1 and that sodium has one electron in its last orbit. So, the valence electron of sodium is one.
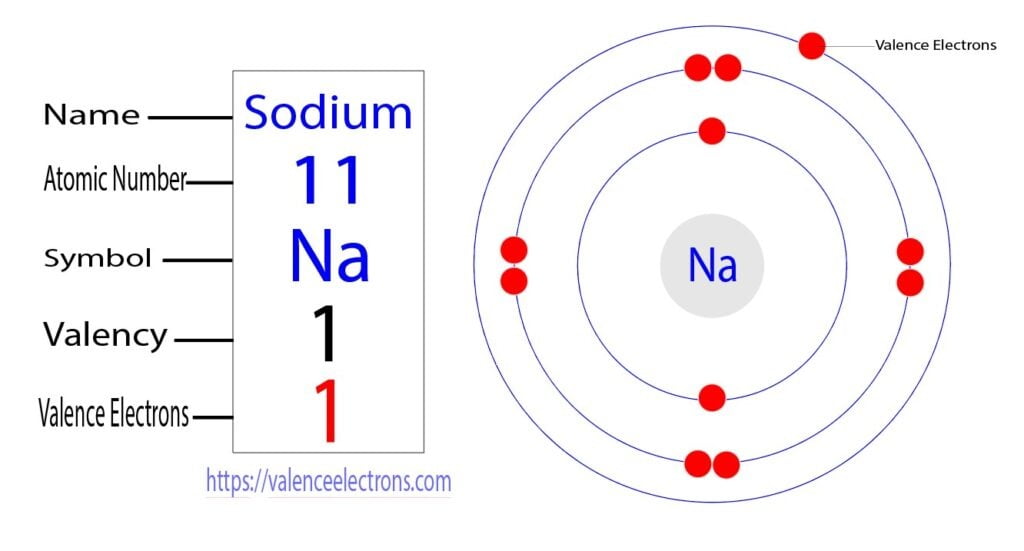
Group-2 elements are called alkaline earth metals. The valence electrons of all the elements in this group are two. For example, the electron configuration of magnesium shows that there are two electrons in the last orbit of magnesium.
Therefore, the group of magnesium is two. The valence electrons of all the elements in this group, including magnesium are two.
Group-3 to 12 elements are called transition metals. The valence electrons of these elements vary by group. To determine the valence electrons of transition elements must know the electron configuration of that element. However, the valence electrons of transition elements range from 3 to 12.
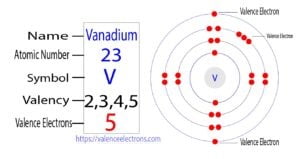
For example, the electron configuration of vanadium shows that there are a total of five electrons in the last orbit of vanadium. So, the valence electrons of vanadium are five. Vanadium is a transition element.
The number of valence electrons in all the elements of group-13 is three. Group-13 is also called the Boron Group. The electron configuration of boron atom shows that there are three electrons in the last orbit of the boron. The valence electrons of all the elements of group-13 including boron are three.
The first element of group-14 is carbon. Therefore, group number fourteen is also called the carbon group. The electron configuration of carbon shows that carbon has a total of four electrons in its final orbit.
So, the valence electrons of carbon are 4. From this, we can understand that the valence electron of all the elements of group-14 is 4.
The first element of group-15 is nitrogen. Therefore, this group is also called the nitrogen group. An element of this group is phosphorus. The electron configuration of phosphorus shows that It has a total of five electrons in its final orbit.
So, the valence electrons of phosphorus are five. The valence electrons of all the elements in this group, including nitrogen, are five.
The valence electrons of all the elements of group-16 are six. Group number sixteen is called the chalcogens group. The first element in this group is oxygen. Selenium is an element of group-16. The atomic number of selenium is 34.
The electron configuration of selenium shows that the last shell has a total of six electrons. So, the valence electrons of selenium are six. All the elements of group-16 have six valence electrons.
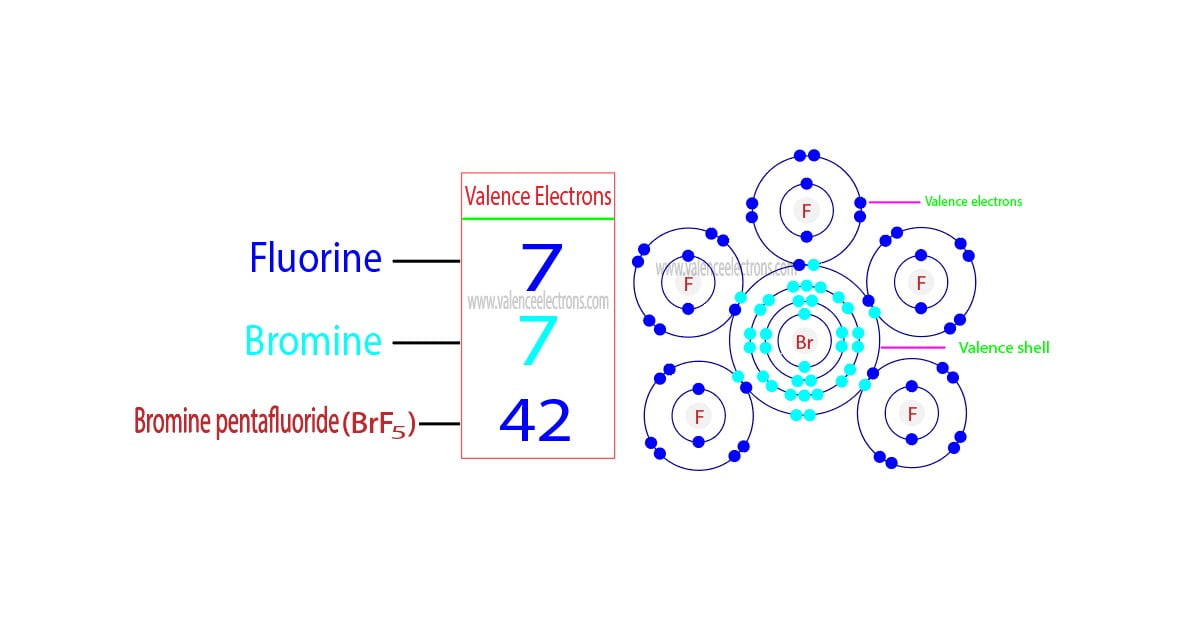
Group-17 is called the halogen group. The elements in this group are fluorine, chlorine, bromine, iodine, and astatine.
The valence electrons of the elements of group-17 are seven. The electron configuration of bromine shows that it has a total of seven electrons in its last shell.
Arranging the electrons of the other elements in this group shows that there are a total of seven electrons in the last shell of each element. Therefore, the valence electrons of all the elements in the halogen group are seven.
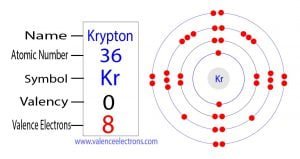
The elements of group-18 are inert elements. These elements do not easily participate in any chemical reaction. These elements remain in the gaseous state at normal temperatures. For these reasons, the elements of Group-18 are called Noble gas.
The octaves of these elements are full except for helium. That is, the last shell contains eight electrons. For example, the atomic number of argon is eighteen. That is, the argon atom has a total of eighteen electrons.
The electron configuration of argon shows that the last shell of an argon atom has a total of eight electrons. So, we can say that the number of valence electrons in the element of group-18 is eight. Only two valence electrons of helium.
Determination of valence electrons through block
The periodic table is divided into four blocks by electron configuration. The blocks are s, p, d, f. The specific valence electrons of any element cannot be determined by the block.
However, it is possible to determine the maximum and minimum valence electrons of the elements in that block. For example, the s-block contains a total of fourteen elements. The valence electrons of these elements are limited to one or two.
Arranging the electrons of the s-block elements shows that the electron configuration ends in the s-orbital. For example, the electron configuration of calcium implies that the electron configuration ends in the s-orbital and the last shell has a total of two electrons.
So, the valence electrons of calcium are two. The p-block consists of a total of six groups from 13 to 18. The number of maximum valence electrons in the elements of this block is eight and the number of minimum valence electrons is three.
The electron configuration of the p-block elements shows that the electron configuration ends in a p-orbital. Therefore, these elements are called p-block elements. For example, the electron configuration of aluminum implies that the electron configuration ends in a p-orbital and the last shell has a total of three electrons.
So, the valence electrons of aluminum are three. The d-block consists of all the elements from group-3 to 12. These elements are called transition elements. The valence electrons of these elements range from 3 to 12.
From the electron configuration of the d-block elements, it is understood that the electron configuration ends in the d-orbital. Therefore, these elements are called d-block elements. For example, the electron configuration of cobalt shows that the electron configuration ends in a d-orbital and that the last shell has a total of nine electrons.
Therefore, the valence electrons of cobalt are nine. The elements in the lanthanides and actinides series are called f-block elements. The valence electrons of these elements range from 3 to 16.
What is the importance of valence electrons in chemical reactions and bond formation?
The electrons in the last shell of the atom participate in chemical reactions and bond formation. Valence electrons participate in both ionic and covalent bonds. The last shell of an element has 1, 2, or 3 electrons, those elements are called metals.
These elements leave electrons and turn into positive ions. Elements that have 5, 6, or 7 electrons in the last shell are called non-metals. All these elements receive electrons and turn into negative ions.
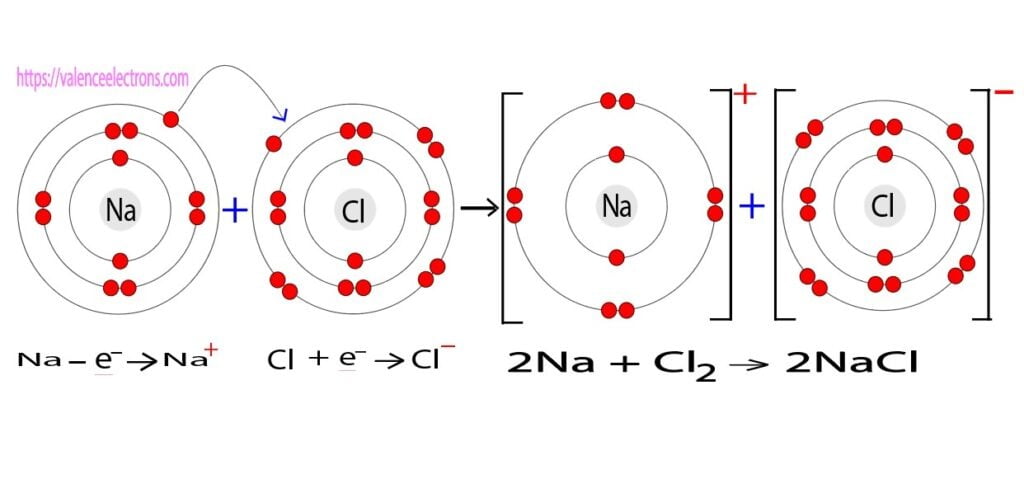
The bond formed by the exchange of electrons between metals and non-metals is called an ionic bond. For example, sodium and chlorine combine to form sodium chloride through ionic bonding.
Sodium has one valence electron and chlorine has seven valence electrons. Sodium leaves an electron in its last shell and chlorine accepts that electron. As a result, sodium chloride bonds are formed.
Again, an oxygen atom has six valence electrons and a hydrogen atom has one valence electron. Oxygen and hydrogen are both non-metallic elements. Hydrogen and oxygen together form covalent bonds through electron share.
The last shell of an oxygen atom has six electrons. The oxygen atom completes the octave by sharing electrons with the two hydrogen atoms.
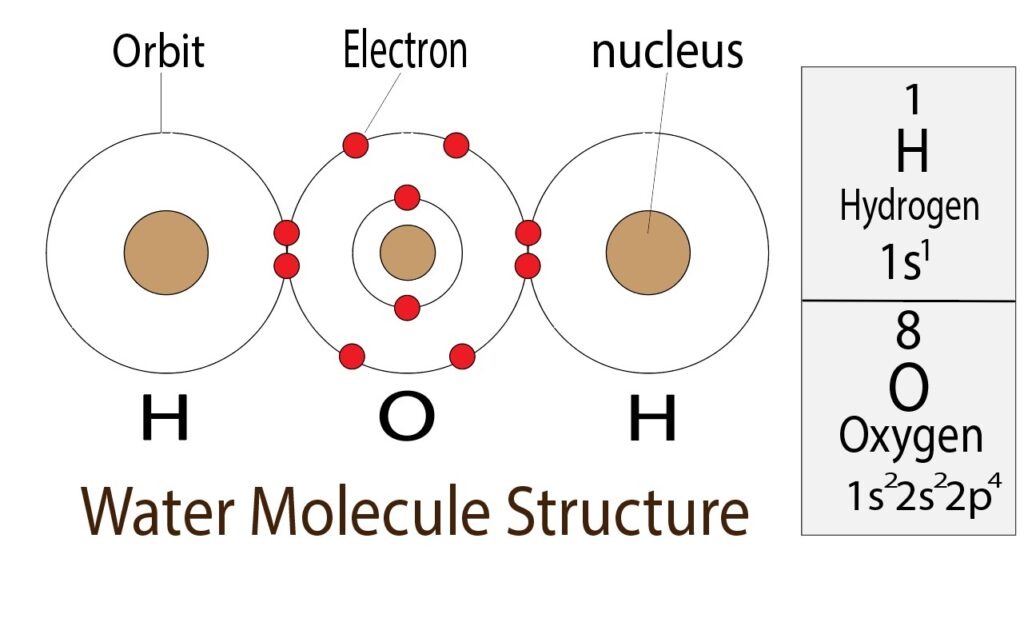
On the other hand, hydrogen acquires the electron configuration of helium and comes to a stable state. In this way, hydrogen and oxygen make water(H2O) through electron sharing.
Determination of valence electrons of exceptional elements
The electron configuration of copper is somewhat exceptional. The atomic number of copper elements is 29. That is, the copper atom has a total of 29 electrons. Like other elements, the electron configuration of copper is 1s2 2s2 2p6 3s2 3p6 4s2 3d9.
In this case, the valence electrons of copper are two. But this electron configuration in copper is not correct. The d-orbital can hold a maximum of ten electrons. From the electron configuration of copper, we can see that it has nine electrons in its d-orbital.
The previous orbital(4s) has two electrons. An electron is transferred from the 4s orbital to the d-orbital. The d-orbital becomes stable as a result of the transfer of an electron. As a result, the electron configuration of copper changes to 1s2 2s2 2p6 3s2 3p6 4s1 3d10.
From the correct electron configuration of copper, we can see that there is an electron in the last orbit. So, the valence electron of copper is 1. However, the valence electrons of copper can be easily determined by following the Bohr principle.
Characteristics of valence electron
- The total number of electrons in the last shell of an element after electron configuration is called the valence electron.
- The valence electrons of the main group elements are in the last shell.
- The valence electrons of the transition element may be in the inner orbital.
- Inert elements have eight valence electrons.
- Characteristic determination of the element can be done through valence electrons.
- The group and block of elements can be determined by valence electrons.
- The electrical conductivity of an element can be determined by valence electrons.
- Valence electrons participate in chemical reactions and bond formation.
- The valency of an element can be determined by valence electrons.