Electron Configuration for Carbon (C, C4−): Full Guide
Carbon is the 6th element in the periodic table and its symbol is ‘C’. In this article, I have discussed in detail how to easily write the complete electron configuration of carbon.
What is the electron configuration of carbon?
The total number of electrons in carbon is six. These electrons are arranged according to specific rules in different orbitals.
The arrangement of electrons in carbon in specific rules in different orbits and orbitals is called the electron configuration of carbon.
The electron configuration of carbon is [He] 2s2 2p2, if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
- Electron configuration through orbit (Bohr principle)
- Electron configuration through orbital (Aufbau principle)
Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund’s principle, and Pauli’s exclusion principle.
Carbon atom electron configuration through orbit
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there.
The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit]
K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2.
| Shell Number (n) | Shell Name | Electrons Holding Capacity (2n2) |
| 1 | K | 2 |
| 2 | L | 8 |
| 3 | M | 18 |
| 4 | N | 32 |
For example,
- n = 1 for K orbit.
The maximum electron holding capacity in K orbit is 2n2 = 2 × 12 = 2. - For L orbit, n = 2.
The maximum electron holding capacity in L orbit is 2n2 = 2 × 22 = 8. - n=3 for M orbit.
The maximum electrons holding capacity in M orbit is 2n2 = 2 × 32 = 18. - n=4 for N orbit.
The maximum electrons holding capacity in N orbit is 2n2 = 2 × 42 = 32.
Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. The atomic number is the number of electrons in that element.
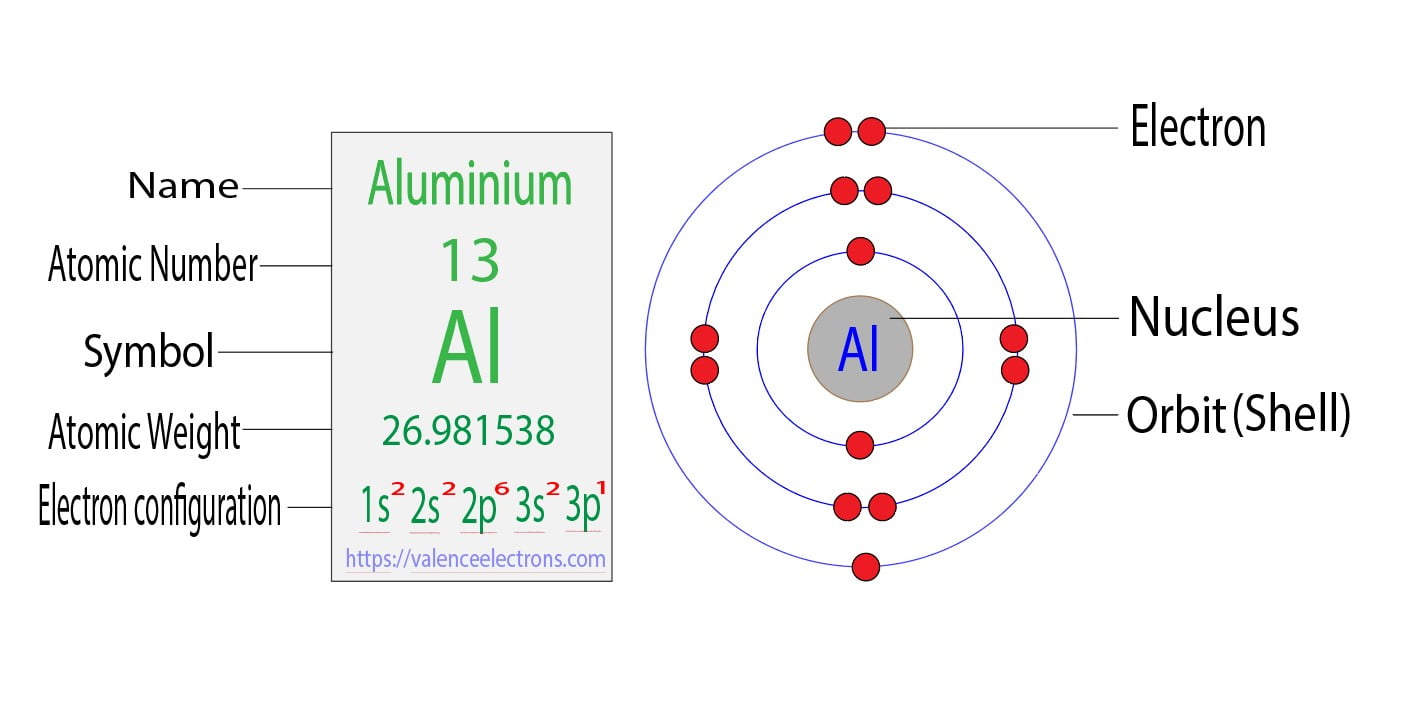
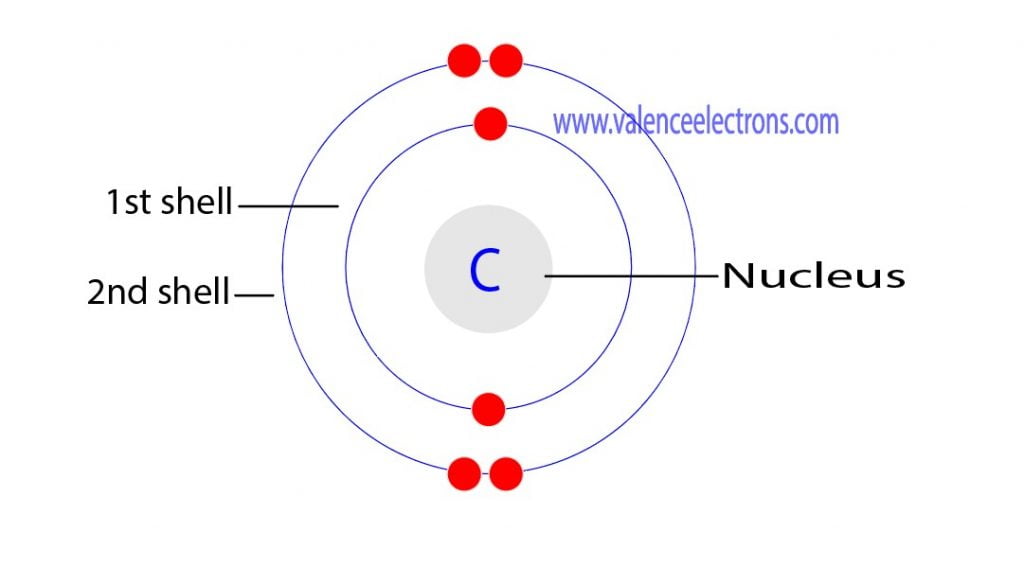
The atomic number of carbon is 6. That is, the number of electrons in carbon is 6. Therefore, a carbon atom will have two electrons in the first shell and four in the 2nd shell.
Therefore, the order of the number of electrons in each shell of the carbon(C) atom is 2, 4. Electrons can be arranged correctly through orbits from elements 1 to 18.
The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram.
Electron configuration of carbon through orbital
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital.
The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, and f.
| Orbit Number | Value of ‘l’ | Number of subshells | Number of orbital | Subshell name | Electrons holding capacity | Electron configuration |
| 1 | 0 | 1 | 1 | 1s | 2 | 1s2 |
| 2 | 0 1 | 2 | 1 3 | 2s 2p | 2 6 | 2s2 2p6 |
| 3 | 0 1 2 | 3 | 1 3 5 | 3s 3p 3d | 2 6 10 | 3s2 3p6 3d10 |
| 4 | 0 1 2 3 | 4 | 1 3 5 7 | 4s 4p 4d 4f | 2 6 10 14 | 4s2 4p6 4d10 4f14 |
For example,
- If n = 1,
(n – 1) = (1–1) = 0
Therefore, the value of ‘l’ is 0. So, the sub-energy level is 1s. - If n = 2,
(n – 1) = (2–1) = 1.
Therefore, the value of ‘l’ is 0, 1. So, the sub-energy levels are 2s, and 2p. - If n = 3,
(n – 1) = (3–1) = 2.
Therefore, the value of ‘l’ is 0, 1, 2. So, the sub-energy levels are 3s, 3p, and 3d. - If n = 4,
(n – 1) = (4–1) = 3
Therefore, the value of ‘l’ is 0, 1, 2, 3. So, the sub-energy levels are 4s, 4p, 4d, and 4f. - If n = 5,
(n – 1) = (n – 5) = 4.
Therefore, l = 0,1,2,3,4. The number of sub-shells will be 5 but 4s, 4p, 4d, and 4f in these four subshells it is possible to arrange the electrons of all the elements of the periodic table.
| Sub-shell name | Name source | Value of ‘l’ | Value of ‘m’ (0 to ± l) | Number of orbital (2l+1) | Electrons holding capacity 2(2l+1) |
| s | Sharp | 0 | 0 | 1 | 2 |
| p | Principal | 1 | −1, 0, +1 | 3 | 6 |
| d | Diffuse | 2 | −2, −1, 0, +1, +2 | 5 | 10 |
| f | Fundamental | 3 | −3, −2, −1, 0, +1, +2, +3 | 7 | 14 |
The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell and seven in the f-subshell. Each orbital can have a maximum of two electrons.
The sub-energy level ‘s’ can hold a maximum of two electrons, ‘p’ can hold a maximum of six electrons, ‘d’ can hold a maximum of ten electrons, and ‘f’ can hold a maximum of fourteen electrons.

Aufbau is a German word, which means building up. The main proponents of this principle are scientists Niels Bohr and Pauli. The Aufbau method is to do electron configuration through the sub-energy level.
The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital.
The energy of an orbital is calculated from the value of the principal quantum number ‘n’ and the azimuthal quantum number ‘l’. The orbital for which the value of (n + l) is lower is the low energy orbital and the electron will enter that orbital first.
| Orbital | Orbit (n) | Azimuthal quantum number (l) | Orbital energy (n + l) |
| 3d | 3 | 2 | 5 |
| 4s | 4 | 0 | 4 |
Here, the energy of 4s orbital is less than that of 3d. So, the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full.
The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d.
The first two electrons of carbon enter the 1s orbital. The s-orbital can have a maximum of two electrons. Therefore, the next two electrons enter the 2s orbital.
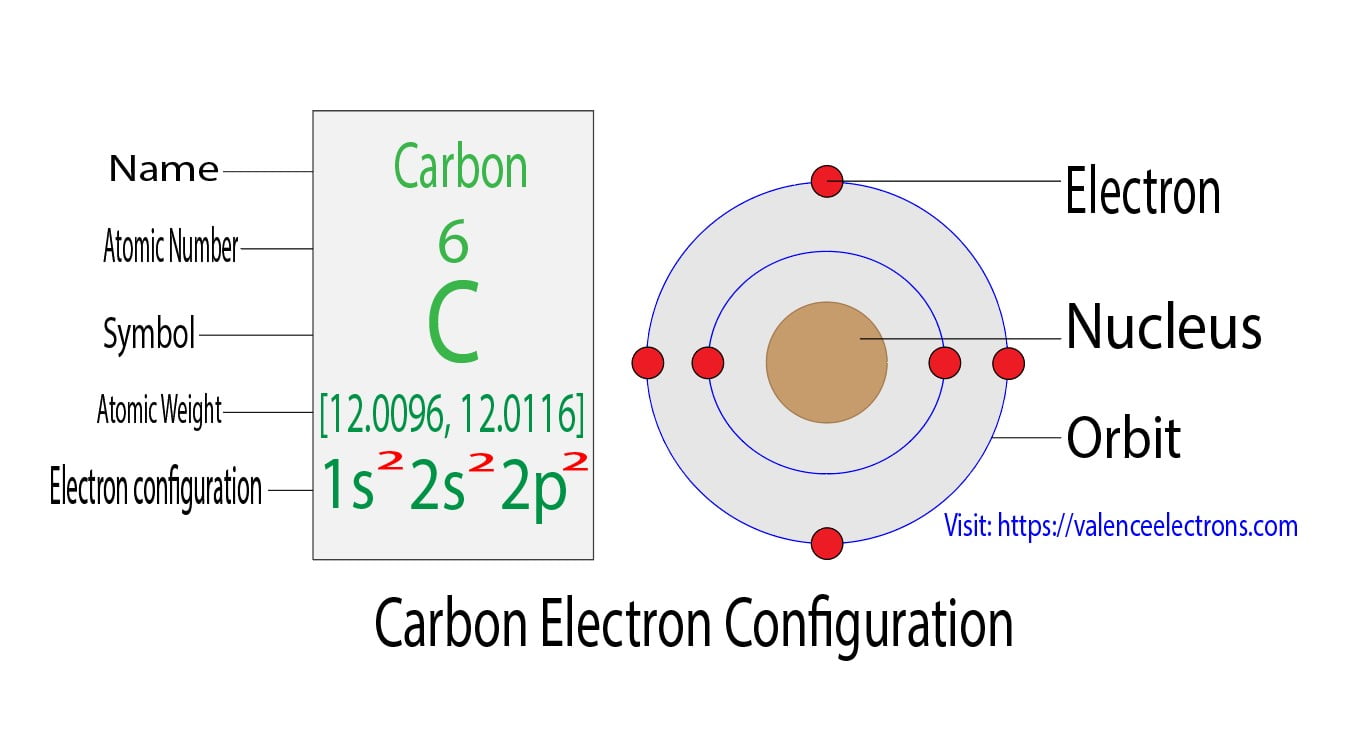
The p-orbital can have a maximum of six electrons. So, the remaining two electrons enter the 2p orbital. Therefore, the carbon complete electron configuration will be 1s2 2s2 2p2.
Note: The unabbreviated electron configuration of carbon is [He] 2s2 2p2. When writing an electron configuration, you have to write serially.
Video for Carbon Electron Configuration
Electron configuration of carbon in the excited state
Atoms can jump from one orbital to another in an excited state. This is called a quantum jump.
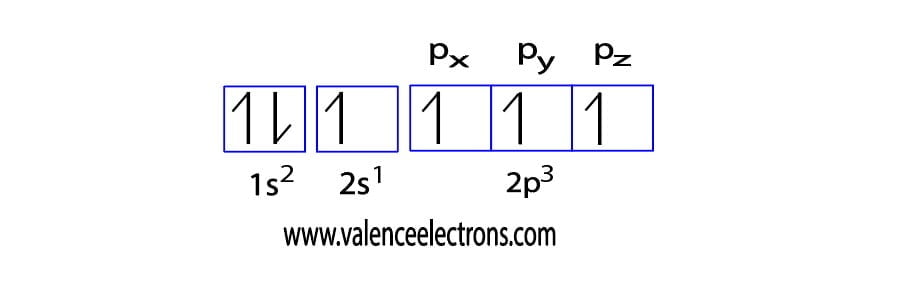
The ground state electron configuration of carbon is 1s2 2s2 2p2. We already know that the p-subshell has three orbitals. The orbitals are px, py, and pz and each orbital can have a maximum of two electrons.
In the carbon ground-state electron configuration, the two electrons of the 2p orbital are located in the px, py orbitals and the spin of the two electrons is the same.
Then the correct electron configuration of carbon in the ground state will be 1s2 2s2 2px1 2py1. Here, carbon has two unpaired electrons. Therefore, the valency of carbon is 2.
Also, the valency of an element is determined by electron configuration in the excited state. When the carbon atom is excited, then the carbon atom absorbs energy. As a result, an electron in the 2s orbital jumps to the 2pz orbital.
Therefore, the electron configuration of carbon(C*) in an excited state will be 1s2 2s1 2px1 2py1 2pz1. Here, carbon has four unpaired electrons. In this case, the valency of carbon is 4.
Carbide ion(C4−) electron configuration
The electron configuration shows that the last shell of carbon has four electrons. Therefore, the valence electrons of carbon are four. The elements that receive electrons and form bonds are called anion.
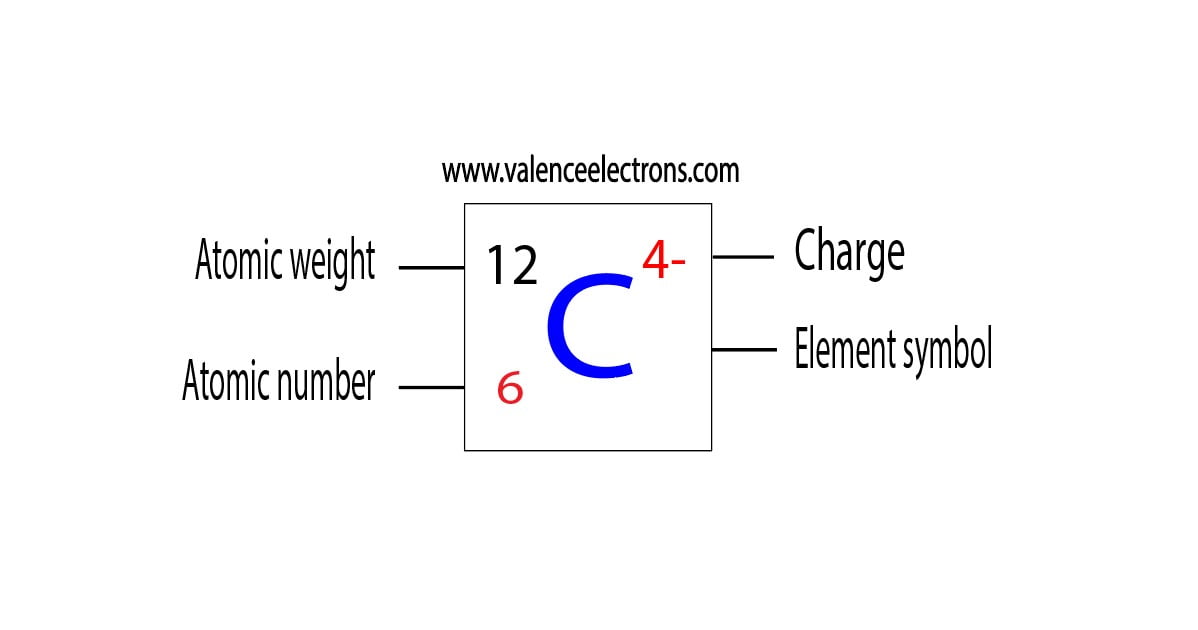
During the formation of a bond, the last shell of carbon receives four electrons and turns into a carbide ion(C4−). That is, carbon is an anion element.
C + 4e– → C4–
Here, the electron configuration of carbide ion(C4−) is 1s2 2s2 2p6. This electron configuration shows that the carbide ion(C4−) acquired the electron configuration of neon and it achieves a stable electron configuration.
Carbon exhibits +4, +2, -4 oxidation state. The oxidation state of the element changes depending on the bond formation.
How to determine the group and period of carbon through the electron configuration?
The last orbit of an element is the period of that element. The electron configuration of the carbon atom shows that the last orbit of the carbon atom is 2. So, the period of carbon is 2.
On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. But in the case of p-block elements, group diagnosis is different.
To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit.
The total number of electrons in the last orbit of the carbon(C) atom is four. That is, the group number of carbon is 4 + 10 = 14. Therefore, we can say that the period of the carbon element is 2 and the group is 14.
How to determine the block of carbon through the electron configuration?
The elements in the periodic table are divided into four blocks based on the electron configuration of the element. The block of elements is determined based on the electron configuration of the element.
If the last electron enters the p-orbital after the electron configuration of the element, then that element is called the p-block element.
The electron configuration shows that the last electron of carbon enters the p-orbital. Therefore, carbon is the p-block element.
Activation sequence of carbon atom
The elements of group-14 are relatively less active. However, the activity of the element increases as it moves from the top to the bottom of the group.
Carbon is the first element of group-14. Carbon is the number one element in group-14. For this, the activation of carbon atoms is very low.
Reaction structure of carbon atom
The carbon atom reacts with the oxygen atom to form oxide compounds.
C + O2 → CO
C + O2 → CO2
In general, the oxides of elements with higher oxidation values are more acidic than the oxides of elements with lower oxidation values. CO is a neutral oxide, but CO2 is an acidic oxide.
CO2 + 2NaOH → Na2CO3 + H2O
CO2 behaves like an acidic oxide.
2C + O2 → 2CO
Here, CO reveals the behavior of the neutral oxide.
The element carbon of group-14 reacts with water to produce [CO + H2] water gas.
C + H2O (1300 C) → [CO + H2]
Carbon atoms react with halogen to form tetrahalide compounds.
- C + 2F2 → CF4
- C + 2Cl2 → CCl4
- C + 2Br2→ CBr4
- C + 2I2 → CI4
The carbon atom reacts with hydrogen to produce methane gas.
C + 2H2 → CH4
Humid analysis of carbon halide
The halide compounds of the 14th group elements, especially the humid analyzed chloride, are of a slightly different nature. In the case of humid analysis, the empty d-orbital of the atom plays a major role.
The second period and 14-group element is carbon. No d-orbital is present at the valence level of the carbon atom. Therefore, CCl4 is not humid analyzed.
Exceptional properties of carbon
The first element of the 14-group is carbon. The 14-group exhibits the exception of carbon from other elements. The exceptions to carbon are shown below-
- The ionic size of carbon atoms is unusually small.
- The electronegativity of carbon atoms is high.
- Carbon is a high ionic potential element.
- The d-orbital is missing in the valence chamber.
- Carbon variant diamonds are very hard.
- The melting point and boiling point of carbon atoms are higher than other elements.
- The valence level of a carbon atom has 2 s-orbitals and 2 p-orbitals. When active, the s-orbital has 1 and the p-orbital has 3 electrons. The maximum valence(valency) of carbon is 4. Since the other elements in the group have d-orbitals present, they can extend the valence up to 6. For example, in the case of silicon (SiF6)2– but [CF6)2– is not possible.
- Small size and high electrical negative element. For this, carbon atoms form single bonds, double bonds, and tri-bonds with other atoms and other elements.
- Catenation; This is one of the characteristics of carbon atoms. Carbon atoms join together to form long chains and rings. Such religions are created because of the small size of the carbon atom and the high carbon-carbon bonding strength. Carbon-carbon bonding strength 348kj/mol.
Bonding structure of carbon
Carbon tetrachloride(CCl4) bonding Structure
There are four electrons in the last orbit of a carbon atom. The carbon atom wants to stabilize by taking four more electrons in its last orbit. Again, electron configuration of chlorine atom shows that the last shell of the chlorine atom has seven electrons.
The chlorine atom wants to complete an octave by taking in an electron. Therefore, a carbon atom forms a carbon tetrachloride(CCl4) compound through covalent bonds by sharing electrons with four chlorine atoms.
Methane(CH4) bond formation
The above electron configuration shows that there are four electrons in the last orbit of the carbon atom. Carbon wants to be stable by taking four electrons in its last orbit(shell).
Again, the hydrogen atom wants to be as stable as helium by taking an electron and completing its first orbit. Therefore, a carbon atom shares electrons with four hydrogen atoms to form the methane(CH4)compound through covalent bonding.
Properties of carbon atom
- The atomic number of carbon atoms is 6. The atomic number of an element is the number of electrons and protons in that element. That is, the number of electrons and protons in the carbon atom is six.
- The active atomic mass of the carbon atom is [12.0096, 12.0116].
- Carbon is non-metal.
- The valency of a carbon atom is 2, 4 and the valence electrons of a carbon atom are four.
- Carbon atoms are the 2nd period of the periodic table and an element of the 14-group.
- Carbon is an electrically negative element.
- Carbon is an anion element.
- Carbon atoms form covalent bonds.
- Carbon is the p-block element. The ionic energy value of carbon atoms is higher than that of s-block elements.
- Carbon is thermally conductive and electricity is slightly conductive.
- The melting point of a carbon atom is 3550°C and the boiling point is 4827°C.
- The electronegativity of carbon atoms is 2.55 (Pauling scale).
- The oxidation states of carbon are -4, 2, and 4.
- The atomic radius of a carbon atom is 67 pm.
- The carbon atom van der Waals radius is 170 pm
- Ionization energies of carbon atoms are 1st: 1086.5 kJ/mol, 2nd: 2352.6 kJ/mol, and 3rd: 4620.5 kJ/mol.
- The electron addiction of carbon atoms is –153.9 kJ mol–1
- The covalent radius of the carbon atom is sp3: 77 pm, sp2: 73 pm, sp: 69 pm.
- C = O Bond Length 123 pm, C – O Bond Length 143 pm, C – C Bond Length 154pm, C = C Bond Length 133 pm, C – H (Alken) Bond Length 109pm, C – H (Alkyne) Bond Length 108 pm, C – H (alkyne) 105 pm, C = N bond length 138pm.
FAQs
How do you write the full electron configuration for carbon?
The full electron configuration for carbon is 1s2 2s2 2p2.
How many electrons does carbon have in its outermost energy level?
Carbon has four electrons in its outermost energy level.
Why does carbon have the electron configuration 1s2 2s2 2p2?
Carbon has an atomic number of 6, which means it has six electrons. The electron configuration follows the Aufbau principle, where electrons fill the lowest energy levels first. The 1s orbital is filled with two electrons, followed by the 2s orbital with another two electrons, and finally, the 2p orbital with two more electrons.
What is the significance of carbon’s electron configuration?
Carbon’s electron configuration determines its chemical behavior. With four electrons in its outermost energy level, carbon can form up to four covalent bonds with other atoms, allowing it to participate in a wide range of chemical reactions and form diverse organic compounds.
How does carbon’s electron configuration relate to its position in the periodic table?
Carbon is located in Group 14 (or Group IVA) of the periodic table. Elements in this group have four valence electrons, which correspond to the electrons in the outermost energy level. Carbon’s electron configuration reflects its position as a nonmetallic element with properties that are crucial for life and a variety of industrial applications.
What is the electronic structure of carbon?
The electronic structure of carbon is 2,4, meaning it has 2 electrons in its innermost energy level and 4 electrons in its outermost energy level.
What is the electron configuration of carbon (in its ground state)?
The correct electron configuration of carbon in the ground state is 1s2 2s2 2px1 2py1.
How many energy levels does carbon have?
Carbon has two energy levels. The first energy level, represented by the 1s orbital, can hold a maximum of 2 electrons, while the second energy level, consisting of the 2s and 2p orbitals, can hold a maximum of 8 electrons.