How to Find the Valence Electrons for Indium (In)?
The 49th element of the periodic table is indium. The element of group-13 is indium and its symbol is ‘In’. Indium forms bonds through its valence electrons. This article discusses in detail how…
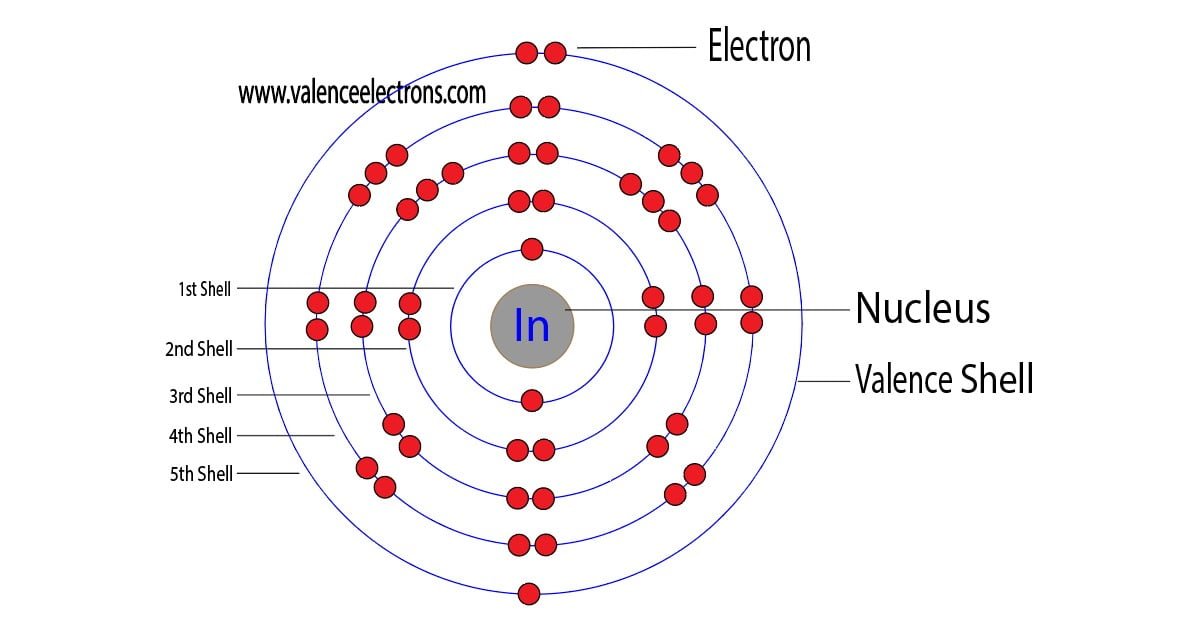
The 49th element of the periodic table is indium. The element of group-13 is indium and its symbol is ‘In’. Indium forms bonds through its valence electrons. This article discusses in detail how…
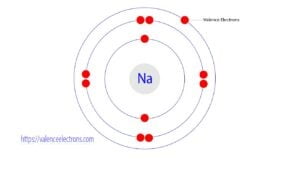
The total number of electrons in the last shell of an atom is called the valence electrons. That is, the total number of electrons in the last orbit of an element after electron…
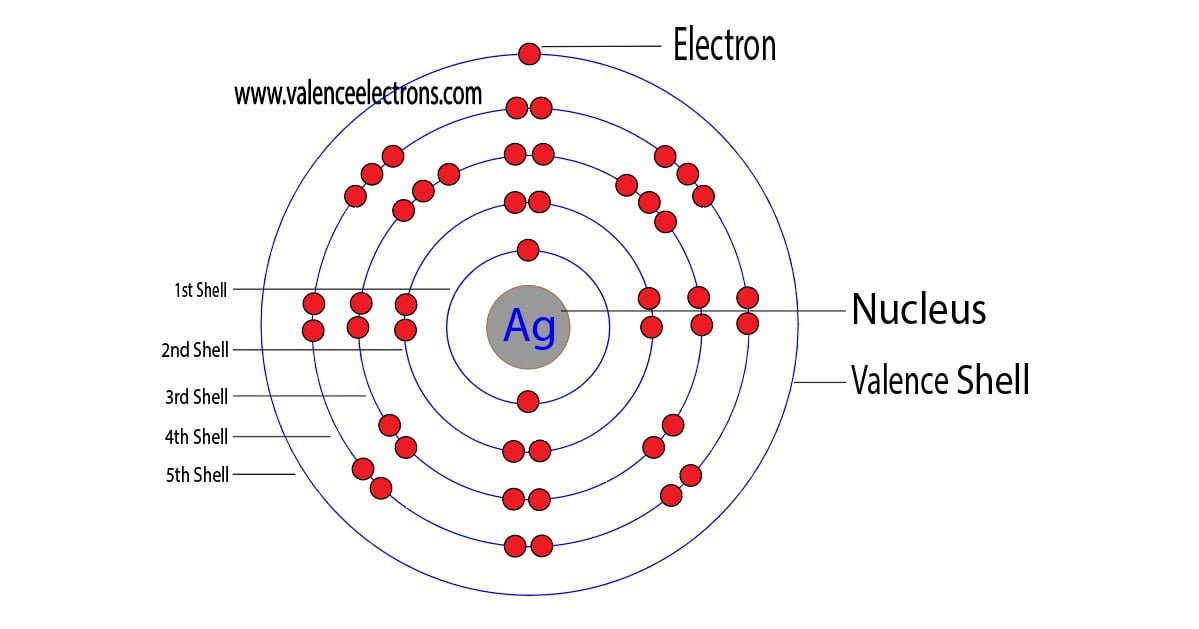
The 47th element of the periodic table is silver. The element of group-11 is silver and its symbol is ‘Ag’. Silver is a d-block element. Therefore, the valence electrons of silver are determined differently….
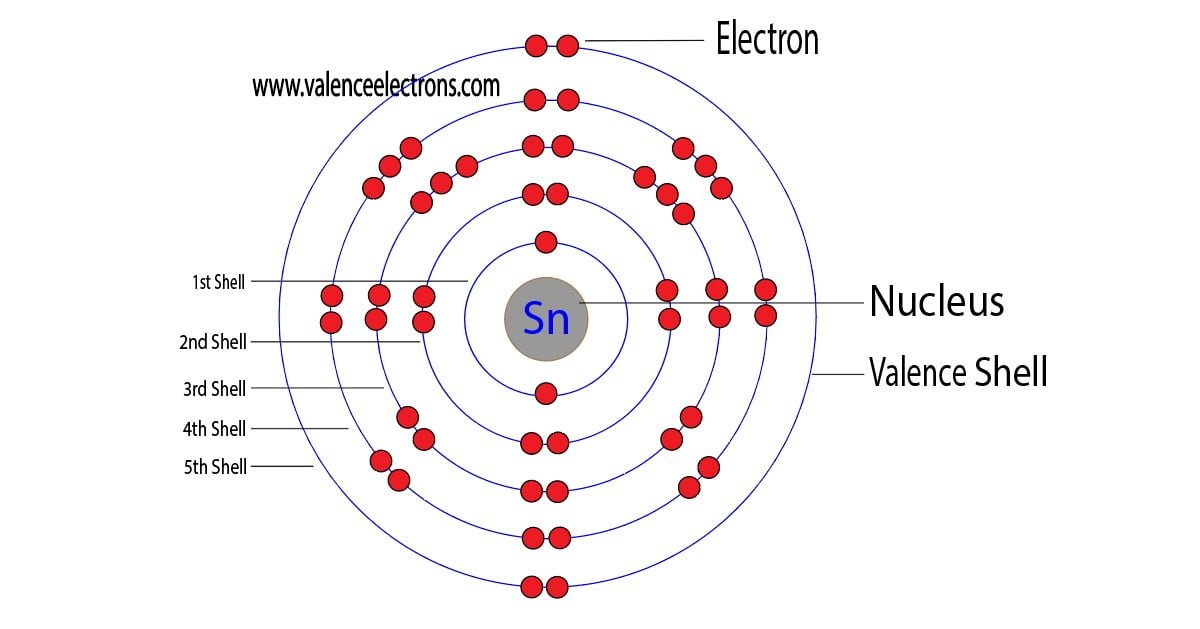
The 50th element of the periodic table is tin. The element of group-14 is tin and its symbol is ‘Sn’. Tin is a post-transition metal and forms bond through its valence electrons. What…
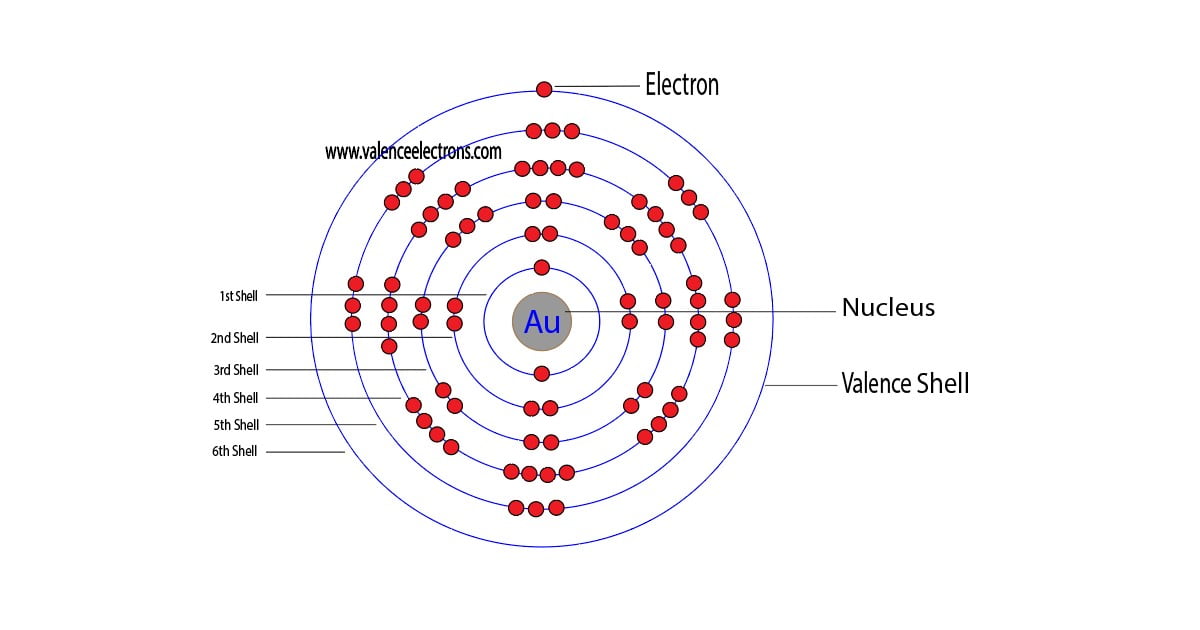
The 79th element of the periodic table is gold. The element of group-11 is gold and its symbol is ‘Au’. Gold is a d-block element. Therefore, the valence electrons of gold are determined differently….
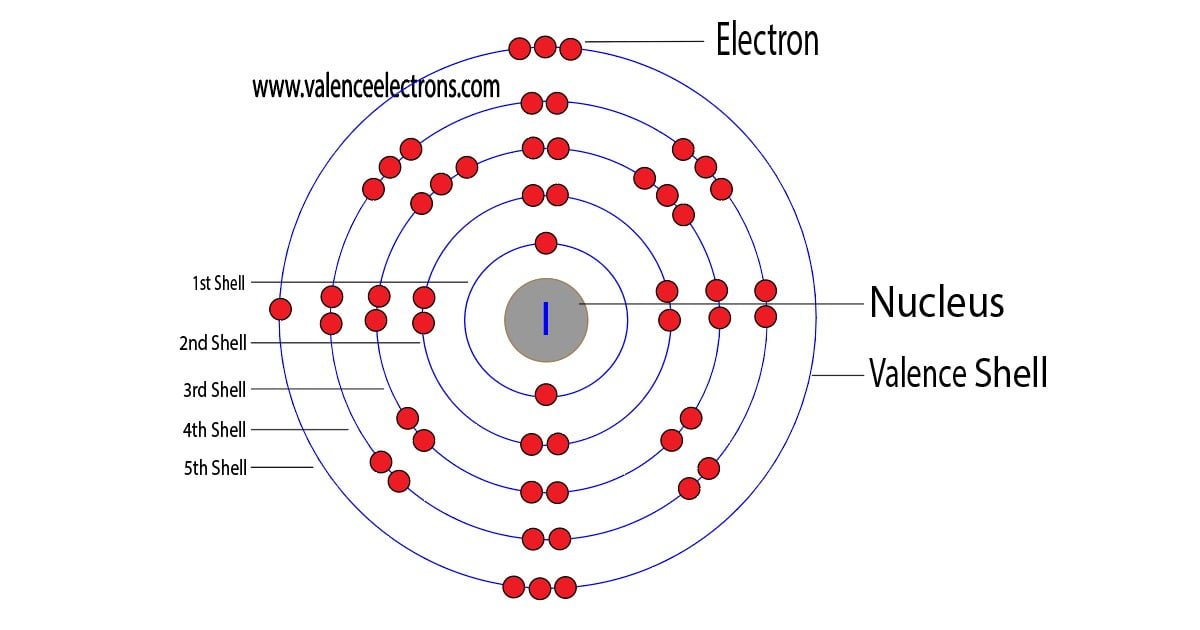
The 53rd element of the periodic table is iodine. The element of group-17 is iodine and its symbol is ‘I’. Iodine is a halogen element and forms bond through its valence electrons. This…
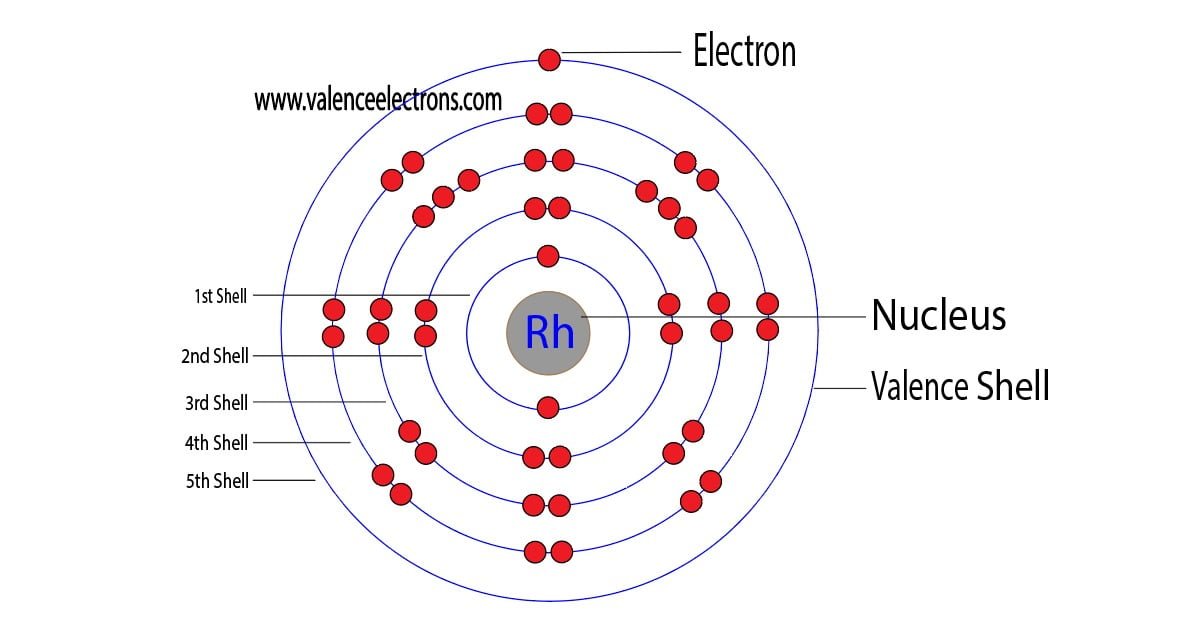
The 45th element in the periodic table is rhodium. The element of group-9 is rhodium and its symbol is ‘Rh’. Rhodium is a d-block element. Therefore, the valence electrons of rhodium are determined differently….
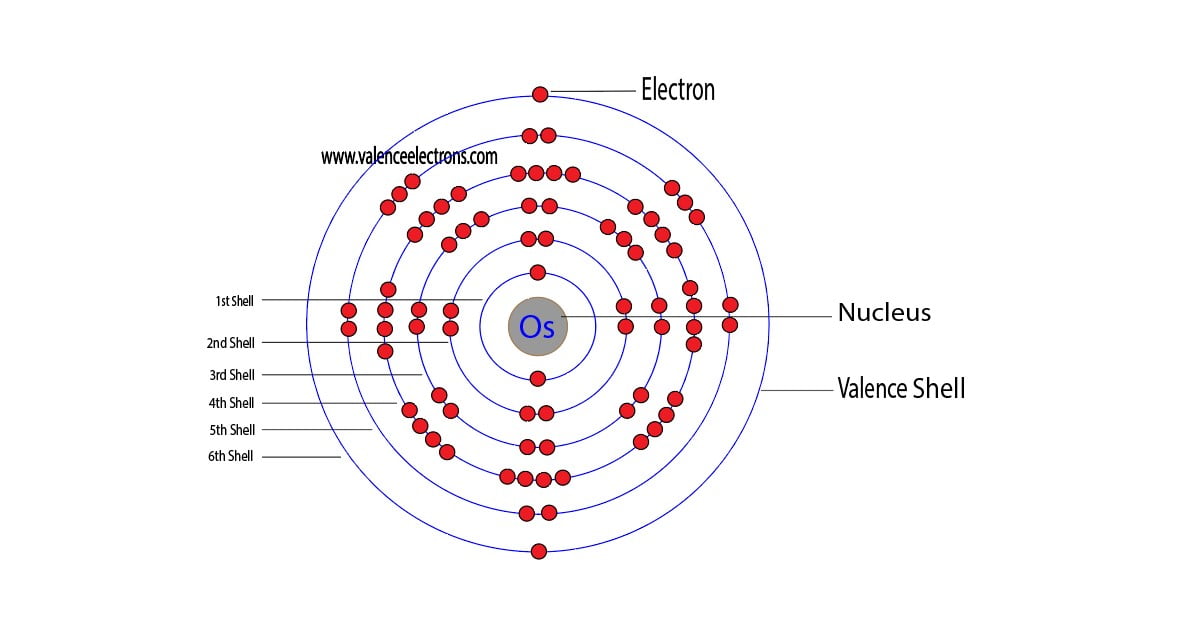
The 76th element in the periodic table is osmium. The element of group-8 is osmium and its symbol is ‘Os’. Osmium is a d-block element. Therefore, the valence electrons of osmium are determined differently….
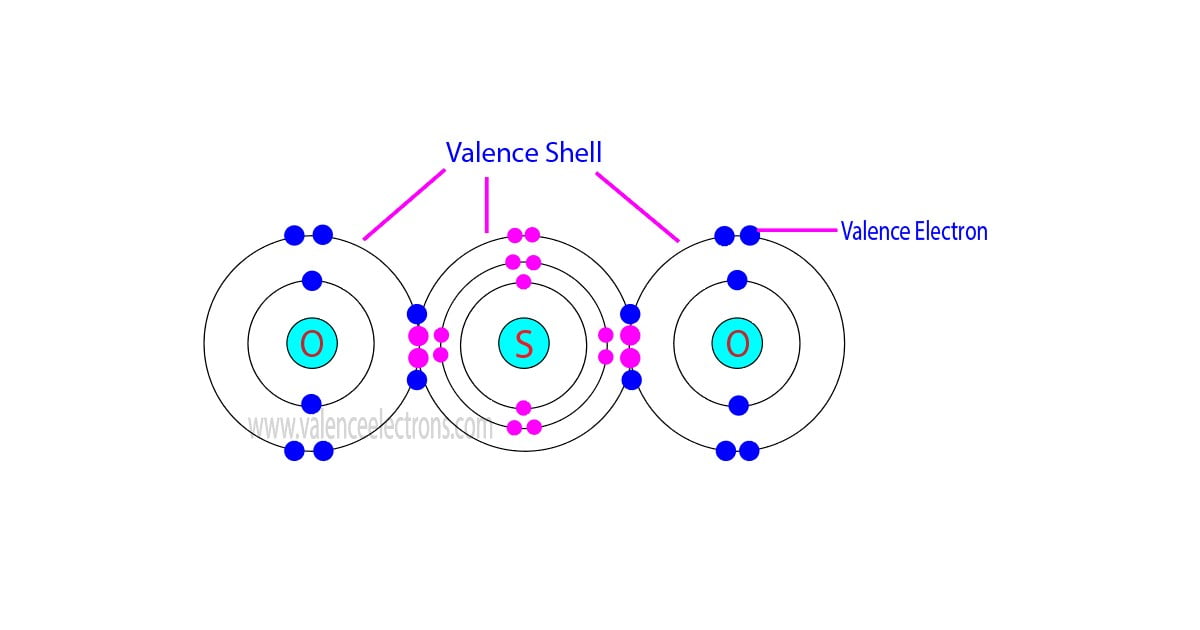
The total number of electrons in the last orbit of an element is called the valence electrons of that element. Two or more elements combine to form compounds. The valence electrons of…
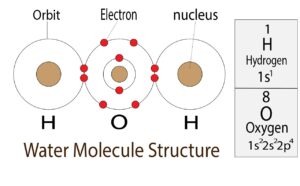
The total number of electrons in the last orbit of an element is called the valence electrons of that element. Two or more elements combine to form compounds. The valence electrons of…
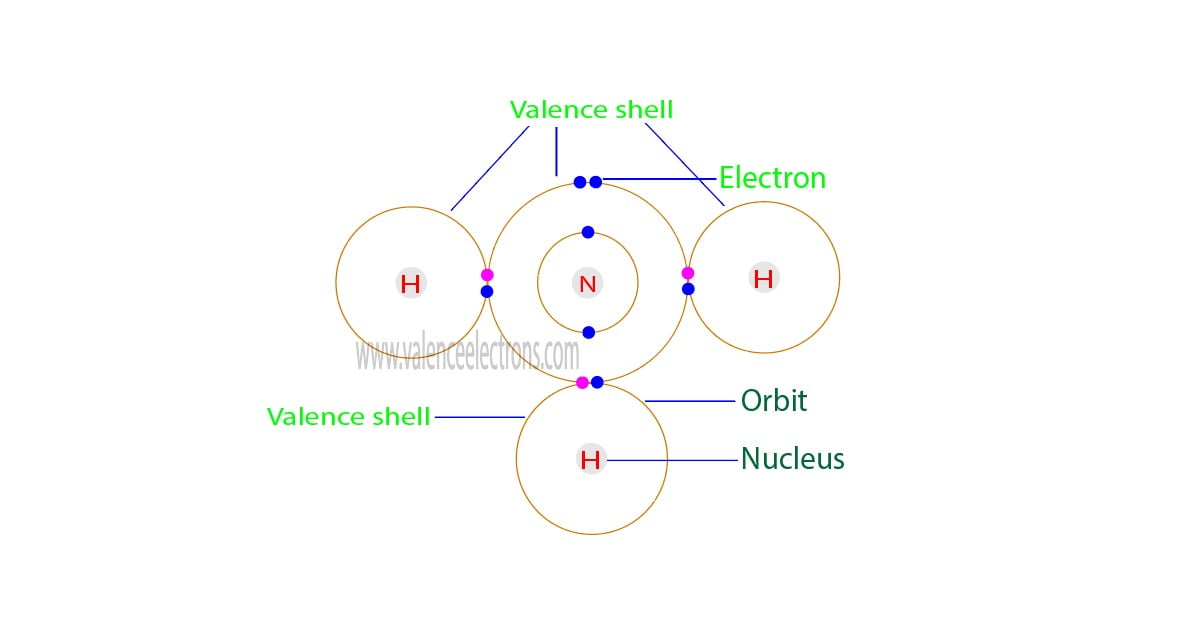
The total number of electrons in the last orbit of an element is called the valence electrons of that element. Two or more elements combine to form compounds. The valence electrons of…
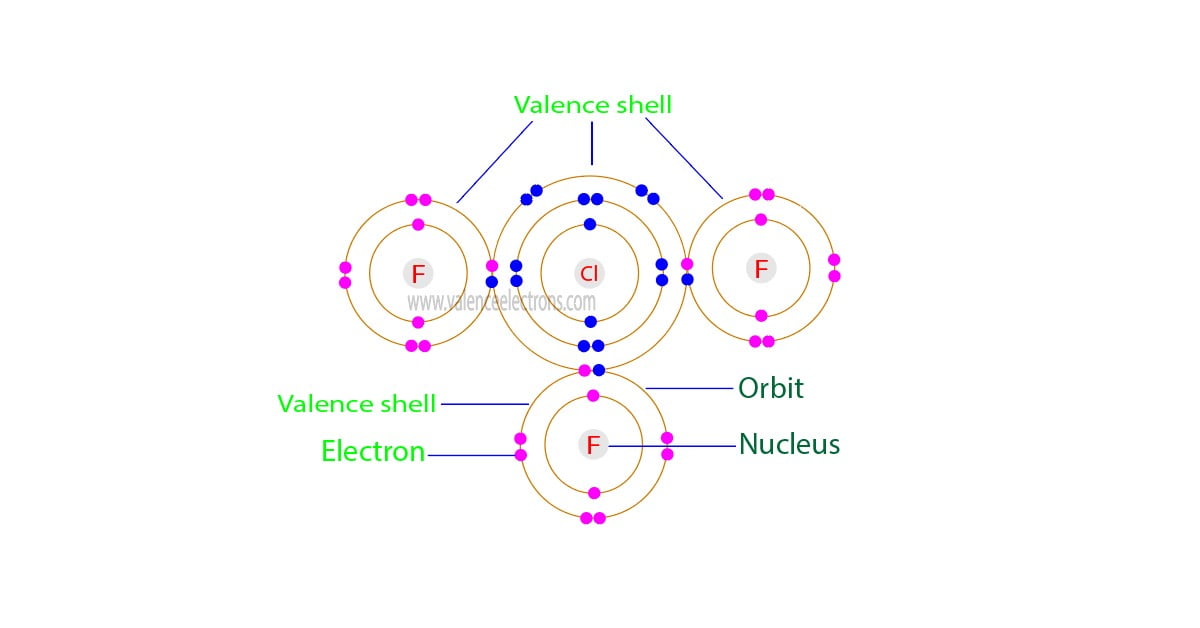
The total number of electrons in the last orbit of an element is called the valence electrons of that element. Two or more elements combine to form compounds. The valence electrons of…
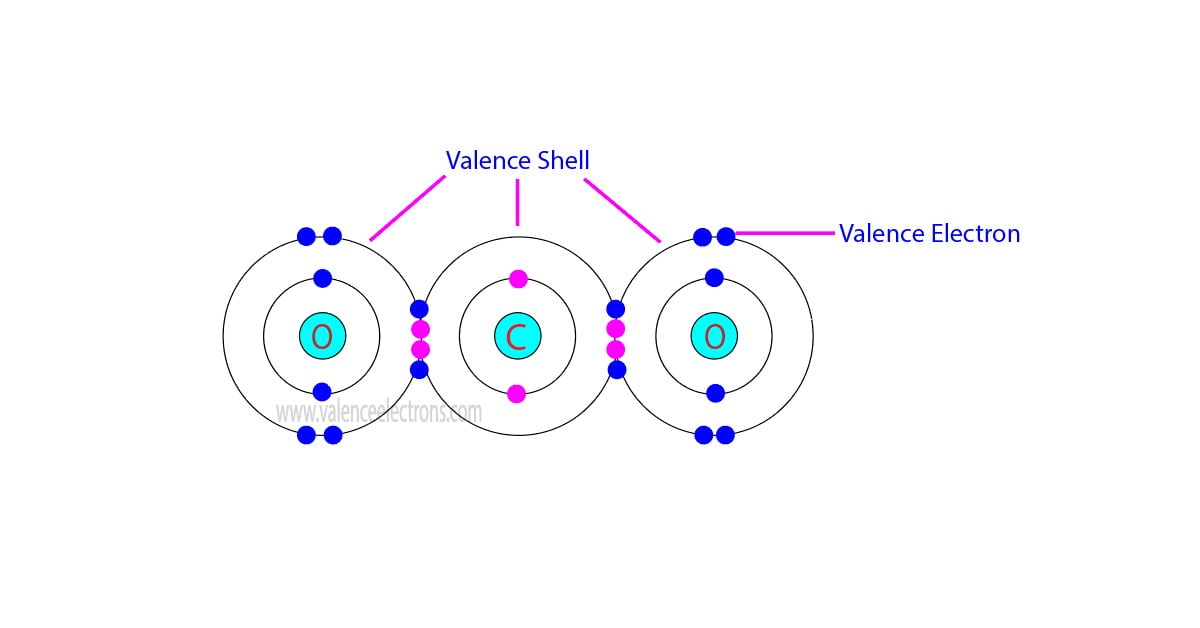
The total number of electrons in the last orbit of an element is called the valence electrons of that element. Two or more elements combine to form compounds. The valence electrons of…
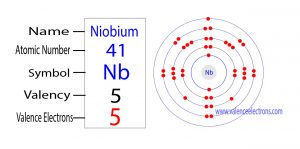
The 41st element in the periodic table is niobium. The element of group-5 is niobium and its symbol is ‘Nb’. Niobium is a d-block element. Therefore, the valence electrons of the niobium…
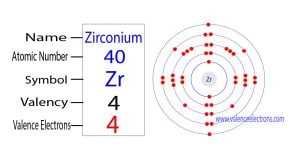
The 40th element in the periodic table is zirconium. The element of group-4 is zirconium and its symbol is ‘Zr’. Zirconium is a d-block element. Therefore, the valence electrons of the zirconium…