How to Find the Valence Electrons for Iodine (I)?
The 53rd element of the periodic table is iodine. The element of group-17 is iodine and its symbol is ‘I’. Iodine is a halogen element and forms bond through its valence electrons.
This article discusses in detail how to easily calculate the number of valence electrons in iodine. Hopefully, after reading this article you will know in detail about this.
What are the valence electrons of iodine?
Iodine is a non-metallic element. The valence electrons are the total number of electrons in the last orbit(shell). The total number of electrons in the last shell after the electron configuration of iodine is called the valence electrons of iodine.
The last shell of iodine has seven electrons. Therefore, the valence electrons of iodine are seven. The valence electrons determine the properties of the element and participate in the formation of bonds.
How do you calculate the number of valence electrons in an iodine atom?
The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electrons without electron configuration.
Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all the elements.
However, valence electrons can be easily identified by arranging electrons according to the Bohr principle. Now we will learn how to determine the valence electron of iodine.
Step-1: Determining the total number of electrons in iodine
The elements in the periodic table are arranged according to their atomic number. Since rhodium is the 53rd element of the periodic table, the atomic number of iodine is 53.
We must always remember that the atomic number and the number of protons of an element are equal. Therefore, an iodine atom contains fifty-three protons.
We must also remember that the number of protons and electrons in an element is equal. Therefore, an iodine atom contains fifty-three electrons in its orbits.
Step-2: Need to do electron configuration of iodine
Step-2 is very important. In this step, the electrons of iodine have to be arranged. We know that iodine atoms have a total of fifty-three electrons.
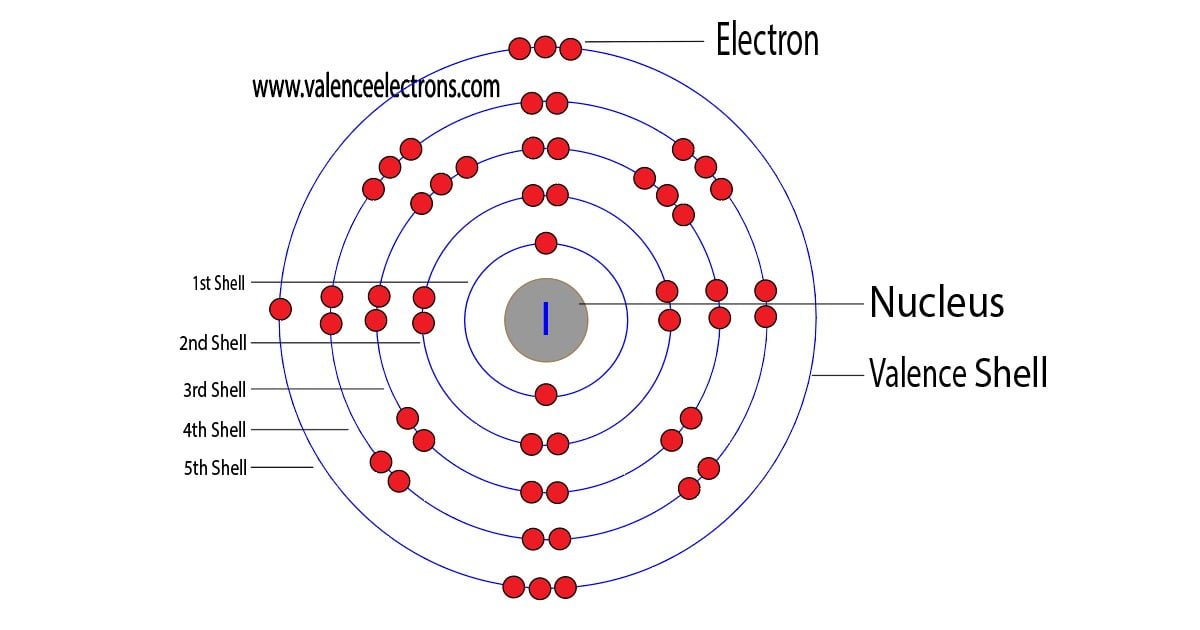
The electron configuration of the iodine shows that the first shell of iodine has two electrons, the second shell has eight electrons, the 3rd shell has eighteen electrons, the 4th shell has also eighteen electrons and the 5th shell has seven electrons.
The number of electrons per shell of iodine is 2, 8, 18, 18, 7.
Step-3: Determine the valence shell and calculate the total electrons
The third step is to diagnose the valence shell. The last shell after the electron configuration is called the valence shell. The total number of electrons in a valence shell is called valence electrons.
The electron configuration shows that the last shell of iodine has seven electrons. Therefore, the valence electrons of iodine are seven.