Electron Configuration for Neon (Ne): Full Explanation
Neon is the 10th element in the periodic table and its symbol is ‘Ne’. In this article, I have discussed in detail how to easily write the complete electron configuration of neon.
What is the electron configuration for neon?
The total number of electrons in neon is ten. These electrons are arranged according to specific rules in different orbitals.
The arrangement of electrons in neon in specific rules in different orbits and orbitals is called the electron configuration of neon.
The electron configuration of neon is [He] 2s2 2p6, if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
- Electron configuration through orbit (Bohr principle)
- Electron configuration through orbital (Aufbau principle)
Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund’s principle, and Pauli’s exclusion principle.
Electron configuration through orbit
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there.
The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit]
K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2.
| Shell Number (n) | Shell Name | Electrons Holding Capacity (2n2) |
| 1 | K | 2 |
| 2 | L | 8 |
| 3 | M | 18 |
| 4 | N | 32 |
For example,
- n = 1 for K orbit.
The maximum electron holding capacity in K orbit is 2n2 = 2 × 12 = 2. - For L orbit, n = 2.
The maximum electron holding capacity in L orbit is 2n2 = 2 × 22 = 8. - n=3 for M orbit.
The maximum electrons holding capacity in M orbit is 2n2 = 2 × 32 = 18. - n=4 for N orbit.
The maximum electrons holding capacity in N orbit is 2n2 = 2 × 42 = 32.
Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. The atomic number is the number of electrons in that element.
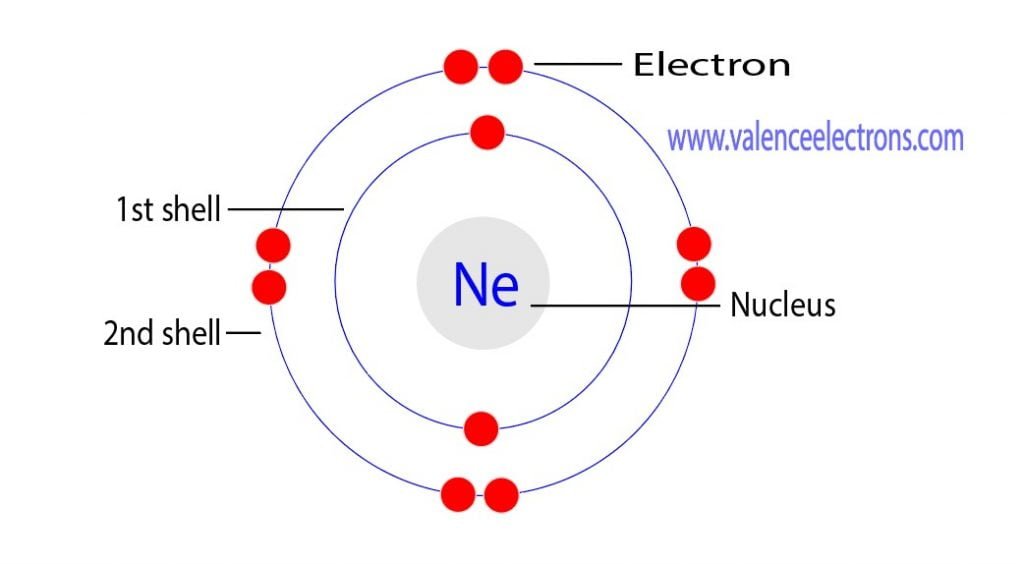
The atomic number of neon is 10. That is, the number of electrons in neon is 10. Therefore, a neon atom will have two electrons in the first shell and eight in the 2nd shell.
Therefore, the order of the number of electrons in each shell of the neon(Ne) atom is 2, 8. Electrons can be arranged correctly through orbits from elements 1 to 18.
The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagrams.
Electron configuration through orbital
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital.
The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, and f.
| Orbit Number | Value of ‘l’ | Number of subshells | Number of orbital | Subshell name | Electrons holding capacity | Electron configuration |
| 1 | 0 | 1 | 1 | 1s | 2 | 1s2 |
| 2 | 0 1 | 2 | 1 3 | 2s 2p | 2 6 | 2s2 2p6 |
| 3 | 0 1 2 | 3 | 1 3 5 | 3s 3p 3d | 2 6 10 | 3s2 3p6 3d10 |
| 4 | 0 1 2 3 | 4 | 1 3 5 7 | 4s 4p 4d 4f | 2 6 10 14 | 4s2 4p6 4d10 4f14 |
For example,
- If n = 1,
(n – 1) = (1–1) = 0
Therefore, the value of ‘l’ is 0. So, the sub-energy level is 1s. - If n = 2,
(n – 1) = (2–1) = 1.
Therefore, the value of ‘l’ is 0, 1. So, the sub-energy levels are 2s, and 2p. - If n = 3,
(n – 1) = (3–1) = 2.
Therefore, the value of ‘l’ is 0, 1, 2. So, the sub-energy levels are 3s, 3p, and 3d. - If n = 4,
(n – 1) = (4–1) = 3
Therefore, the value of ‘l’ is 0, 1, 2, 3. So, the sub-energy levels are 4s, 4p, 4d, and 4f. - If n = 5,
(n – 1) = (n – 5) = 4.
Therefore, l = 0,1,2,3,4. The number of sub-shells will be 5 but 4s, 4p, 4d, and 4f in these four subshells it is possible to arrange the electrons of all the elements of the periodic table.
| Sub-shell name | Name source | Value of ‘l’ | Value of ‘m’ (0 to ± l) | Number of orbital (2l+1) | Electrons holding capacity 2(2l+1) |
| s | Sharp | 0 | 0 | 1 | 2 |
| p | Principal | 1 | −1, 0, +1 | 3 | 6 |
| d | Diffuse | 2 | −2, −1, 0, +1, +2 | 5 | 10 |
| f | Fundamental | 3 | −3, −2, −1, 0, +1, +2, +3 | 7 | 14 |
The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell and seven in the f-subshell. Each orbital can have a maximum of two electrons.
The sub-energy level ‘s’ can hold a maximum of two electrons, ‘p’ can hold a maximum of six electrons, ‘d’ can hold a maximum of ten electrons, and ‘f’ can hold a maximum of fourteen electrons.
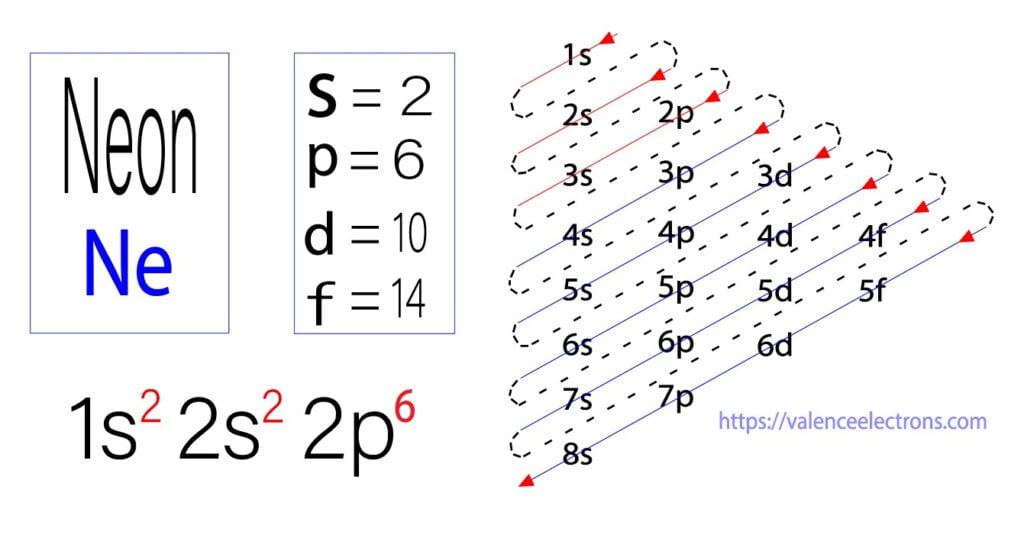
Aufbau is a German word, which means building up. The main proponents of this principle are scientists Niels Bohr and Pauli. The Aufbau method is to do electron configuration through the sub-energy level.
The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital.
The energy of an orbital is calculated from the value of the principal quantum number ‘n’ and the azimuthal quantum number ‘l’. The orbital for which the value of (n + l) is lower is the low energy orbital and the electron will enter that orbital first.
| Orbital | Orbit (n) | Azimuthal quantum number (l) | Orbital energy (n + l) |
| 3d | 3 | 2 | 5 |
| 4s | 4 | 0 | 4 |
Here, the energy of 4s orbital is less than that of 3d. So, the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full.
The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. The first two electrons of neon enter the 1s orbital and the next two electrons enter the 2s orbital.
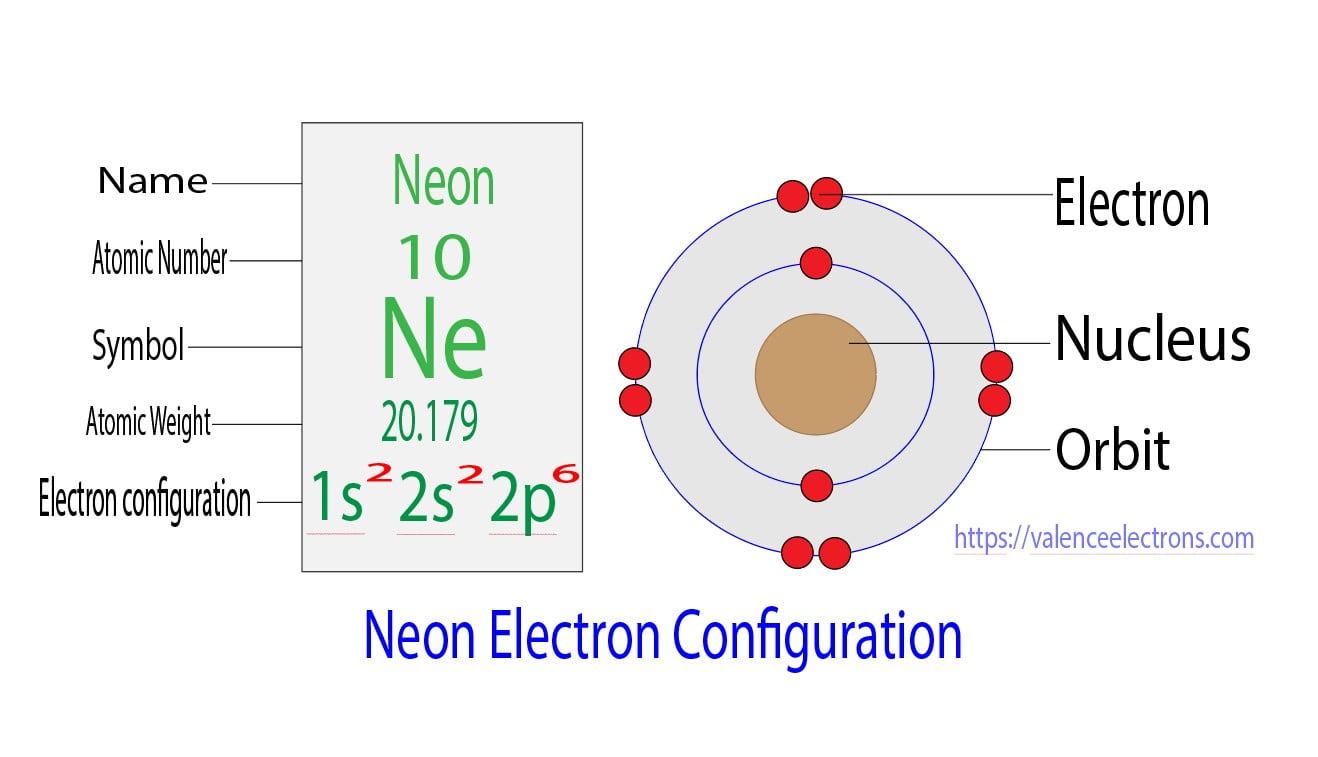
The s-orbital can have a maximum of two electrons. So, the remaining six electrons enter the 2p orbital. Therefore, the neon complete electron configuration will be 1s2 2s2 2p6.
Note: The unabbreviated electron configuration of neon is [He] 2s2 2p6. When writing an electron configuration, you have to write serially.
Video for Neon (Ne) Electron Configuration
How to determine the group and period of neon through the electron configuration?
The last orbit of an element is the period of that element. The electron configuration of the neon atom shows that the last orbit of the neon atom is 2. So, the period of neon is 2.
On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element. But in the case of p-block elements, group diagnosis is different.
To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit.
The total number of electrons in the last orbit of the neon atom is eight. That is, the group number of neon is 8 + 10 = 18. Therefore, we can say that the period of the neon element is 2 and the group is 18.
How to determine the block of neon through the electron configuration?
The elements in the periodic table are divided into four blocks based on the electron configuration of the element. The block of elements is determined based on the electron configuration of the element.
If the last electron enters the p-orbital after the electron configuration of the element, then that element is called the p-block element.
The electron configuration of neon(Ne) shows that the last electron of neon enters the p-orbital. Therefore, neon is the p-block element.
How to determine the valency and valence electrons of neon?
The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an element is the valency of that element.
The electron configuration of neon shows that neon is an inert element. There are eight electrons in the last orbit of a neon atom. The neon atom has no unpaired electrons. Therefore, the valency of the neon atom is 0.
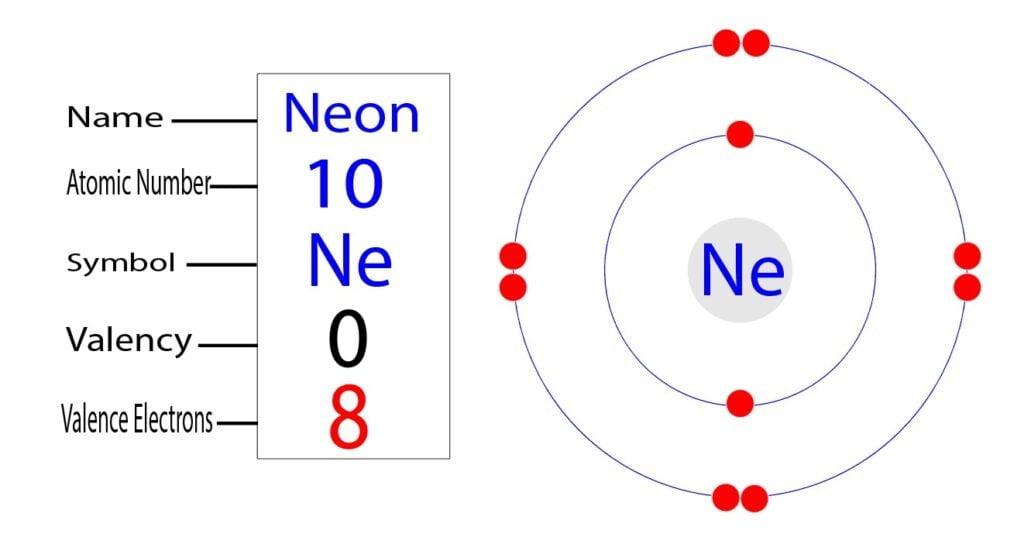
Again, the number of electrons in the last orbit of an element, the number of those electrons is the valence electrons of that element. In the electron configuration, we see that eight electrons exist in the last orbit of the neon.
Therefore, the valence electrons of the neon are eight. Finally, we can say that the valency of the neon is 0, and the valence electrons of the neon are eight.
Reasons for placing neon in group-18 of the periodic table
The electron configuration of neon shows that the number of electrons in the last orbit of the neon atom is eight. We know that the number of electrons in the last orbit of an element is the number of groups in that element.
Accordingly, the group of neon is ten but neon is an inert element. All inert elements are placed in group number 18 in the periodic table. Therefore, neon is placed in group-18 instead of group-10.
Why is neon an inert gas?
The elements in group-18 of the periodic table are inert gases. The inert gases of Group-18 are helium(He), neon(Ne), argon(Ar), krypton(Kr), xenon(Xe), and radon(Rn).
We know that the element in group-18 is neon. The electron configuration of neon shows that the orbit at the end of neon is filled with electrons. Neon does not want to exchange or share any electrons because the last orbit of neon is full of electrons.
And neon does not form any compounds because it does not share any electrons. They do not participate in chemical bonding and chemical reactions.
For this, they are called inert elements. The inert elements are in the form of gases at normal temperatures. For this inert elements are called inert gases. Again for this same reason, inert gas is called a noble gas.
Properties of neon
- The atomic number of neon atoms is 10. The atomic number of an element is the number of electrons and protons in that element. That is, the number of electrons and protons in the neon atom is ten.
- The active atomic mass of the neon atom is 20.1797.
- Neon is an inert element.
- The valency(valence) of a neon atom is zero and the valence electrons of a neon atom are 8.
- Neon atoms are the 2nd period of the periodic table and an element of the 18-group.
- The electron configuration of neon ends in a p-orbital. Therefore, it is a p-block element.
- The melting point of a neon atom is 24.56 K (−248.59 °C, −415.46 °F) and the boiling point is 27.104 K (−246.046 °C, −410.883 °F).
- The value electronegativity of neon atoms is 0.
- The oxidation state of neon is 0.
- Neon atom van der Waals radius is 154 pm.
- Ionization energies of neon atoms are 1st: 2080.7 kJ/mol, 2nd: 3952.3 kJ/mol, 3rd: 6122 kJ/mol.
- The covalent radius of the neon atom is 58 pm.
- Neon atoms do not participate in any chemical reaction.
- The atomic radius of the neon atom is 38pm.
- Neon is normally in the form of gas.
FAQs
What is the full electron configuration for neon?
The full electron configuration for neon is 1s2 2s2 2p6.
What is the electronic structure of neon?
The electronic structure of neon (Ne) is 1s2 2s2 2p6. This means that neon has two electrons in the 1s orbital, two electrons in the 2s orbital, and six electrons in the 2p orbitals. Neon has a completely filled valence shell, making it an inert or noble gas.
How many orbitals does neon have?
Neon has a total of five orbitals: one 1s orbital, one 2s orbital, and three sets of 2p orbitals (2px, 2py, 2pz) with one orbital in each set. Each orbital can hold a maximum of two electrons.
How many unpaired electrons does neon have?
Neon has no unpaired electrons. The electron configuration of neon is 1s2 2s2 2p6, indicating that all of its orbitals are filled with paired electrons. Neon is a noble gas and is known for its stability due to its fully-filled valence shell.
How many total electrons are located in neon’s second energy level?
There are eight electrons located in neon’s second energy level. The second energy level consists of the 2s and 2p orbitals, and it can accommodate a maximum of eight electrons according to the electron capacity rule (2 + 2 + 4 = 8).