How to Find the Valence Electrons for Neon (Ne)?
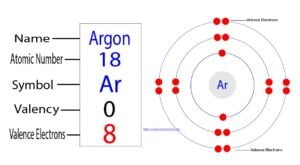
Neon is the tenth element in the periodic table. Neon is an inert element and its symbol is ‘Ne’. Neon atoms do not participate in the formation of any bonds.
What are the valence electrons of neon?
Neon is an element of group-18. The valence electron is the total number of electrons in the last orbit(shell). The total number of electrons in the last shell after the electron configuration of neon is called the valence electrons of neon.
The valence electrons determine the properties of the element and participate in the formation of bonds.
How do you calculate the number of valence electrons in a neon atom?
The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration.
Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all the elements.
However, this article briefly discusses the electron configuration of neon. However, valence electrons can be easily identified by arranging electrons according to the Bohr principle.
Step-1: Determining the total number of electrons in neon
First we need to know the total number of electrons in the neon atom. To know the number of electrons, you need to know the number of protons in neon.
And to know the number of protons, you need to know the atomic number of the neon element.
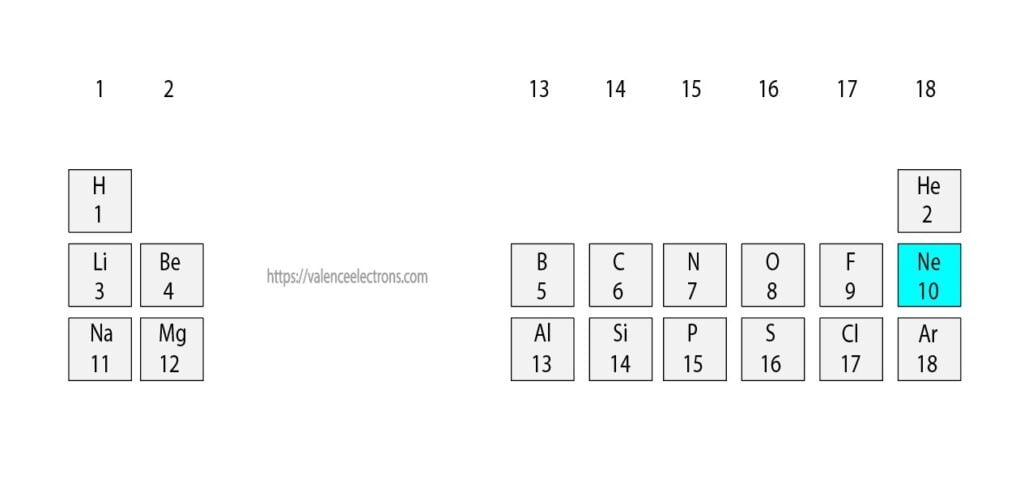
To know the atomic number we need to take the help of a periodic table. It is necessary to know the atomic number of neon(Ne) elements from the periodic table.
The atomic number is the number of protons and electrons equal to protons located outside the nucleus.
That is, we can finally say that there are electrons equal to the atomic number in the neon atom. From the periodic table, we see that the atomic number of neon is 10. That is, a neon atom has a total of ten electrons.
Step-2: Need to do electron configuration of neon
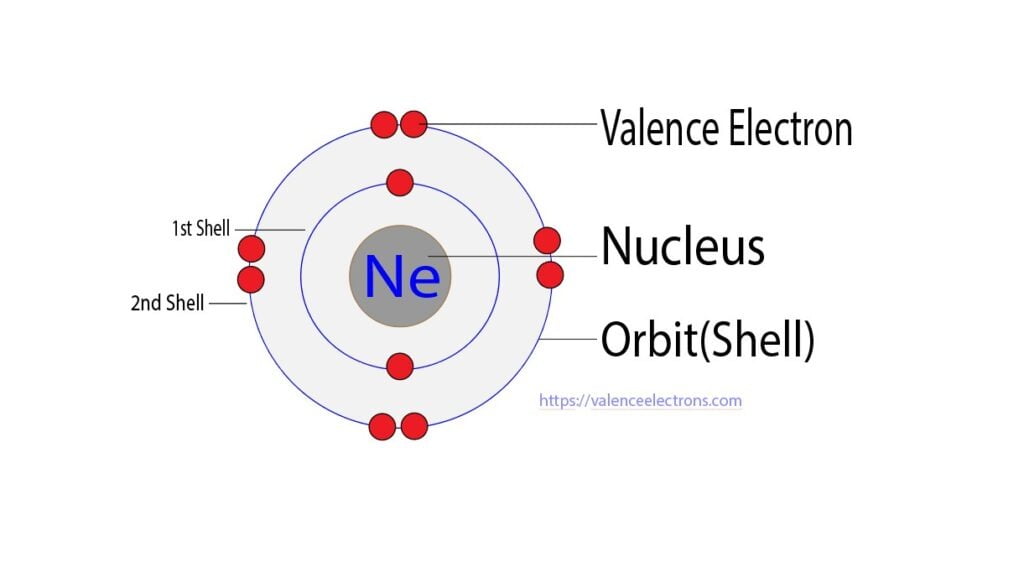
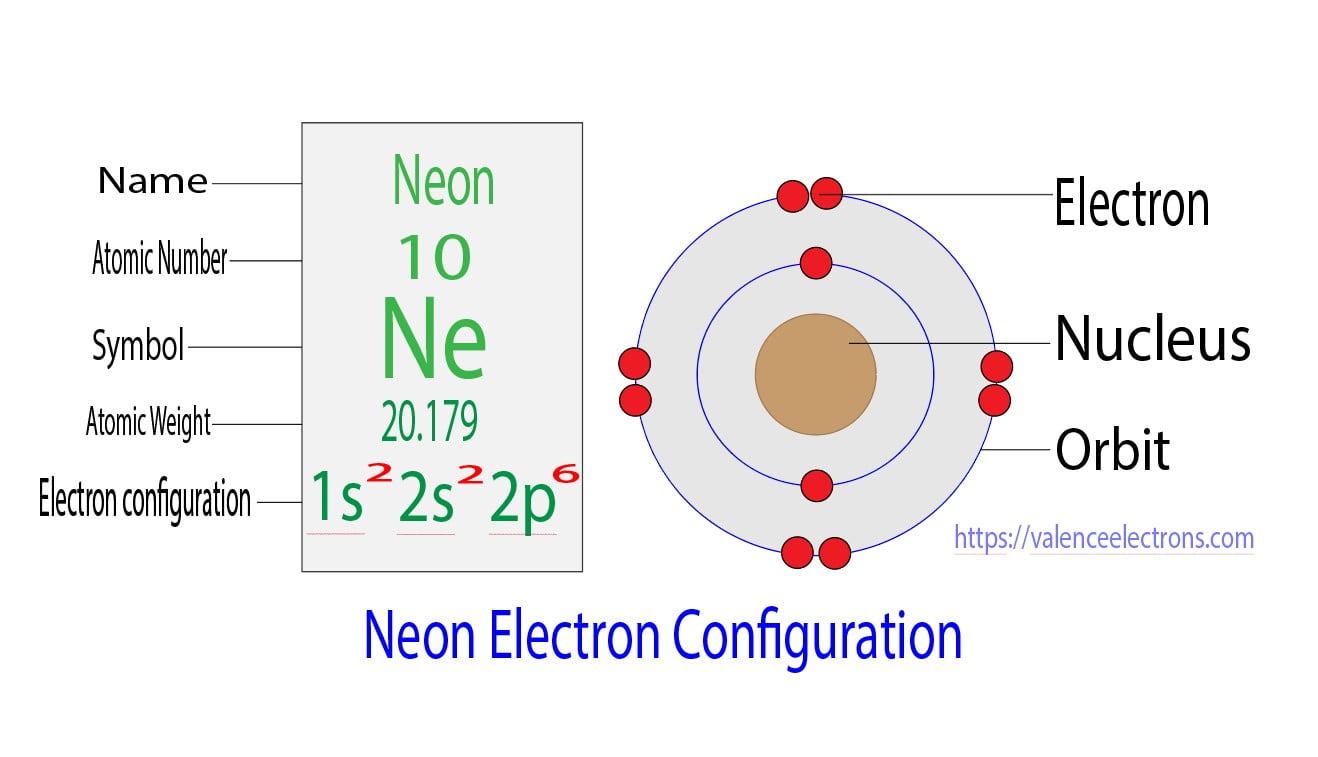
Step 2 is very important. In this step, the electrons of neon have to be arranged. We know that neon atoms have a total of ten electrons.
The electron configuration of neon shows that there are two electrons in the K shell and eight in the L shell. That is, the first shell of neon has two and the second shell has eight electrons.
Step-3: Determine the valence shell and calculate the total electrons
The third step is to diagnose the valence shell(orbit). The last shell after the electron configuration is called the valence shell. The total number of electrons in a valence shell is called a valence electron.
The electron configuration of neon shows that the last shell of neon has eight electrons. Therefore, the valence electrons of neon are eight.
Video for How to find the Valence Electrons for Neon (Ne)
What is the valency of neon?
The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an element is the valency of that element.
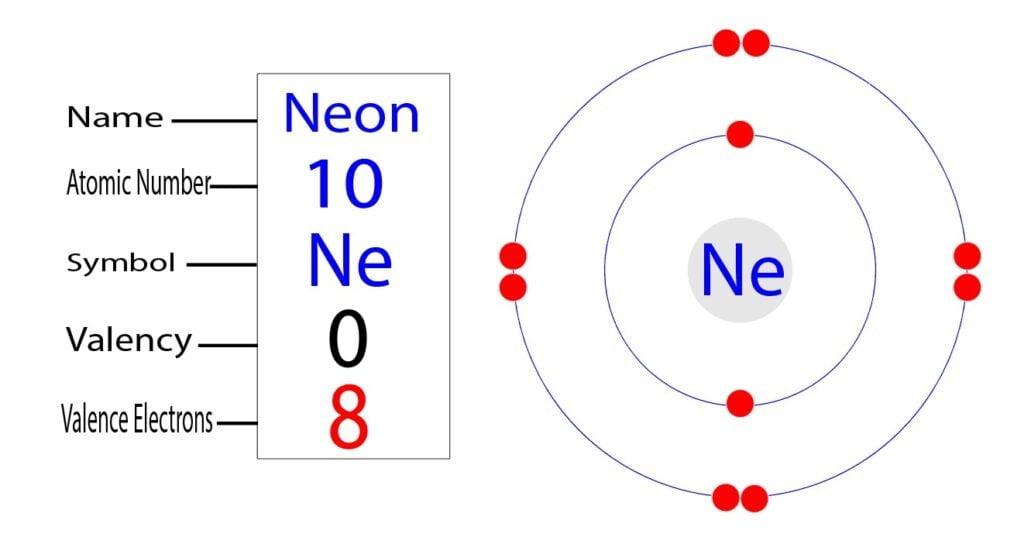
The electron configuration of neon shows that neon is an inert element. There are eight electrons in the last orbit of a neon atom. The neon atom has no unpaired electrons. Therefore, the valency of the neon(Ne) atom is 0.
Reasons for placing neon in group-18 of the periodic table
The electron configuration of neon shows that the number of electrons in the last orbit of the neon atom is 8. We know that the number of electrons in the last orbit of an element is the number of groups in that element.
Accordingly, the group of neon is 8 but neon is an inert element. All inert elements are placed in group number 18 in the periodic table. Therefore, neon is placed in group-18 instead of group-8.
Why is neon an inert gas?
The elements in group-18 of the periodic table are inert gases. The inert gases of Group-18 are helium (He), neon(Ne), argon(Ar), krypton(Kr), xenon(Xe), and radon(Rn).
We know that the element in group-18 is neon(Ne). The electron configuration of neon shows that the orbit at the end of neon is filled with electrons.
Neon does not want to exchange or share any electrons because the last orbit of neon is full of electrons. And neon does not form any compounds because it does not share any electrons.
They do not participate in chemical bonding and chemical reactions. For this, they are called inert elements. The inert elements are in the form of gases at normal temperatures.
For this inert elements are called inert gases. Again for this same reason, inert gas is called a noble gas.