Electron Configuration for Scandium (Sc, Sc3+ ion)
Scandium is the 21st element in the periodic table and its symbol is ‘Sc’. In this article, I have discussed in detail how to easily write the complete electron configuration of scandium.
What is the electron configuration of scandium?
The total number of electrons in scandium is twenty-one. These electrons are arranged according to specific rules in different orbitals.
The arrangement of electrons in scandium in specific rules in different orbits and orbitals is called the electron configuration of scandium.
The electron configuration of scandium is [Ar] 3d1 4s2, if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
- Electron configuration through orbit (Bohr principle)
- Electron configuration through orbital (Aufbau principle)
Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund’s principle, and Pauli’s exclusion principle.
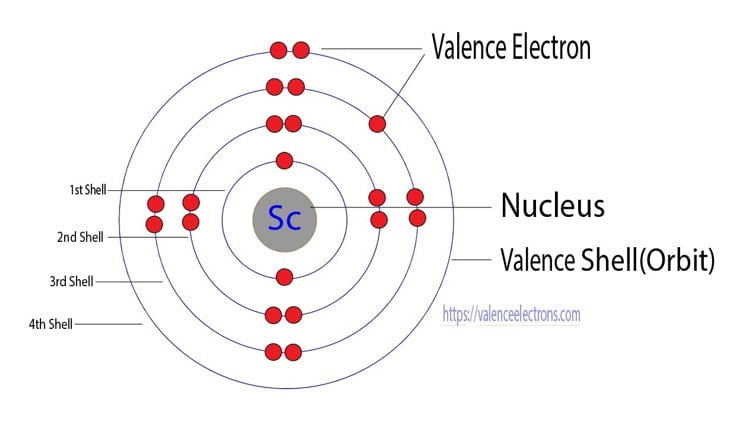
Scandium atom electron configuration through orbit
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there.
The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit]
K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2.
| Shell Number (n) | Shell Name | Electrons Holding Capacity (2n2) |
| 1 | K | 2 |
| 2 | L | 8 |
| 3 | M | 18 |
| 4 | N | 32 |
For example,
- n = 1 for K orbit.
The maximum electron holding capacity in K orbit is 2n2 = 2 × 12 = 2. - For L orbit, n = 2.
The maximum electron holding capacity in L orbit is 2n2 = 2 × 22 = 8. - n=3 for M orbit.
The maximum electrons holding capacity in M orbit is 2n2 = 2 × 32 = 18. - n=4 for N orbit.
The maximum electrons holding capacity in N orbit is 2n2 = 2 × 42 = 32.
Therefore, the maximum electron holding capacity in the first shell is two, the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. The atomic number is the number of electrons in that element.
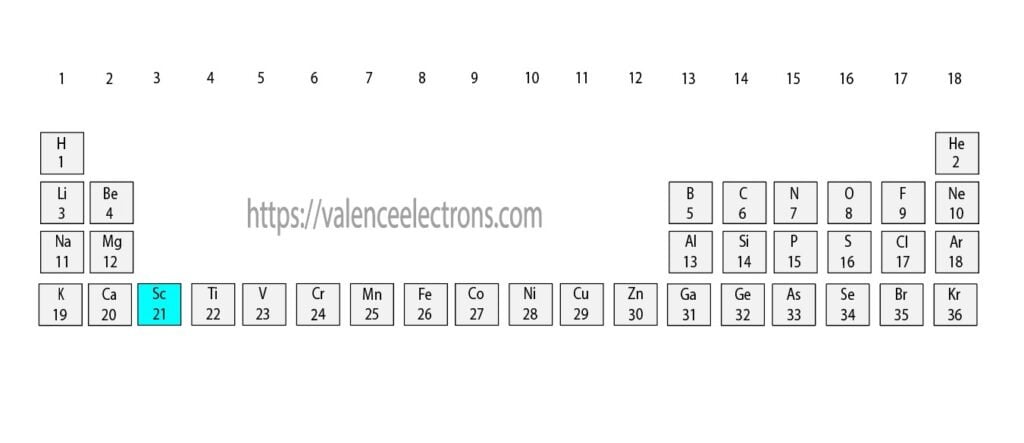
The atomic number of scandium is 21. That is, the number of electrons in scandium is twenty-one. Therefore, the scandium atom will have two electrons in the first shell and eight in the 2nd shell.
According to Bohr’s formula, the third orbit will have eleven electrons but the third orbit of scandium will have nine electrons and the remaining two electrons will be in the fourth orbit.
Therefore, the order of the number of electrons in each shell of the scandium(Sc) atom is 2, 8, 9, 2. Electrons can be arranged correctly through orbits from elements 1 to 18.
The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram.
Electron configuration of scandium through orbital
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital.
The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, and f.
| Orbit Number | Value of ‘l’ | Number of subshells | Number of orbital | Subshell name | Electrons holding capacity | Electron configuration |
| 1 | 0 | 1 | 1 | 1s | 2 | 1s2 |
| 2 | 0 1 | 2 | 1 3 | 2s 2p | 2 6 | 2s2 2p6 |
| 3 | 0 1 2 | 3 | 1 3 5 | 3s 3p 3d | 2 6 10 | 3s2 3p6 3d10 |
| 4 | 0 1 2 3 | 4 | 1 3 5 7 | 4s 4p 4d 4f | 2 6 10 14 | 4s2 4p6 4d10 4f14 |
For example,
- If n = 1,
(n – 1) = (1–1) = 0
Therefore, the value of ‘l’ is 0. So, the sub-energy level is 1s. - If n = 2,
(n – 1) = (2–1) = 1.
Therefore, the value of ‘l’ is 0, 1. So, the sub-energy levels are 2s, and 2p. - If n = 3,
(n – 1) = (3–1) = 2.
Therefore, the value of ‘l’ is 0, 1, 2. So, the sub-energy levels are 3s, 3p, and 3d. - If n = 4,
(n – 1) = (4–1) = 3
Therefore, the value of ‘l’ is 0, 1, 2, 3. So, the sub-energy levels are 4s, 4p, 4d, and 4f. - If n = 5,
(n – 1) = (n – 5) = 4.
Therefore, l = 0,1,2,3,4. The number of sub-shells will be 5 but 4s, 4p, 4d, and 4f in these four subshells it is possible to arrange the electrons of all the elements of the periodic table.
| Subshell name | Name source | Value of ‘l’ | Value of ‘m’ (0 to ± l) | Number of orbital (2l+1) | Electrons holding capacity 2(2l+1) |
| s | Sharp | 0 | 0 | 1 | 2 |
| p | Principal | 1 | −1, 0, +1 | 3 | 6 |
| d | Diffuse | 2 | −2, −1, 0, +1, +2 | 5 | 10 |
| f | Fundamental | 3 | −3, −2, −1, 0, +1, +2, +3 | 7 | 14 |
The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell and seven in the f-subshell. Each orbital can have a maximum of two electrons.
The sub-energy level ‘s’ can hold a maximum of two electrons, ‘p’ can hold a maximum of six electrons, ‘d’ can hold a maximum of ten electrons, and ‘f’ can hold a maximum of fourteen electrons.

Aufbau is a German word, which means building up. The main proponents of this principle are scientists Niels Bohr and Pauli. The Aufbau method is to do electron configuration through the sub-energy level.
The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital.
The energy of an orbital is calculated from the value of the principal quantum number ‘n’ and the azimuthal quantum number ‘l’. The orbital for which the value of (n + l) is lower is the low energy orbital and the electron will enter that orbital first.
| Orbital | Orbit (n) | Azimuthal quantum number (l) | Orbital energy (n + l) |
| 3d | 3 | 2 | 5 |
| 4s | 4 | 0 | 4 |
Here, the energy of 4s orbital is less than that of 3d. So, the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full.
The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d.
The first two electrons of scandium enter the 1s orbital. The s-orbital can have a maximum of two electrons. Therefore, the next two electrons enter the 2s orbital. The p-orbital can have a maximum of six electrons.
So, the next six electrons enter the 2p orbital. The second orbit is now full. So, the remaining electrons will enter the third orbit. Then two electrons will enter the 3s orbital of the third orbit and the next six electrons will be in the 3p orbital.
The 3p orbital is now full. So, the next two electrons will enter the 4s orbital and the remaining one electron will enter the 3d orbital. Therefore, the scandium full electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d1 4s2.
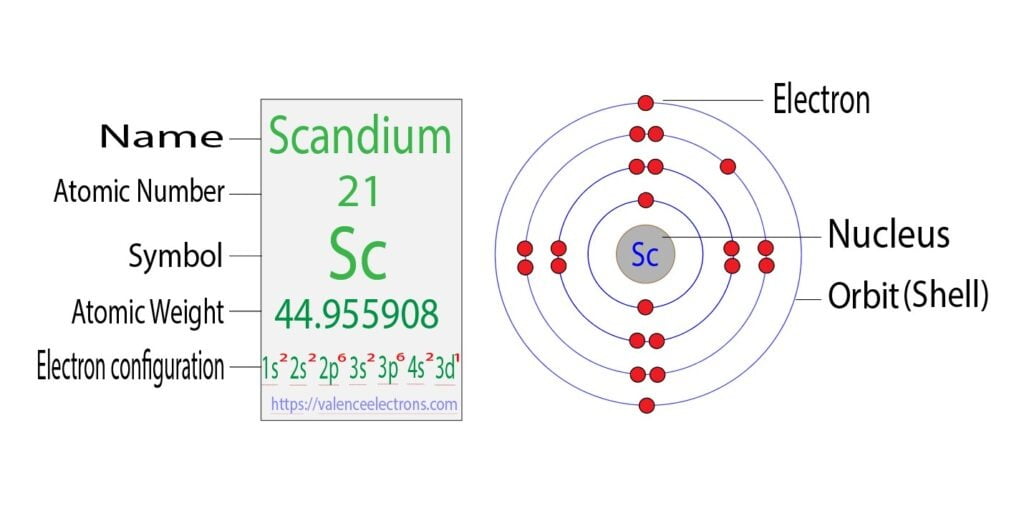
Note: The abbreviated electron configuration of scandium is [Ar] 3d1 4s2. When writing an electron configuration, you have to write serially.
Video for Electron configuration for Scandium (Sc)
Scandium excited state electron configuration
Atoms can jump from one orbital to another orbital in the excited state. This is called quantum jump.
The ground state electron configuration of scandium is 1s2 2s2 2p6 3s2 3p6 3d1 4s2. This electron configuration shows that the last shell of the scandium atom has two electrons.
When scandium atoms are excited, then scandium atoms absorb energy. As a result, an electron in the 4s orbital jumps to the 4px orbital.
We already know that the p-subshell has three orbitals. The orbitals are px, py, and pz and each orbital can have a maximum of two electrons.
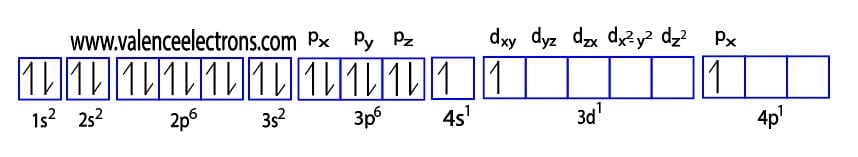
Therefore, the electron configuration of scandium(Sc*) in an excited state will be 1s2 2s2 2p6 3s2 3p6 3d1 4s1 4px1. The valency of the element is determined by electron configuration in the excited state. Here, scandium has three unpaired electrons. So the valency of scandium is 3.
Scandium ion(Sc3+) electron configuration
After the electron configuration, the last shell of scandium has two electrons and the d-orbital has a total of an electron. Therefore, the valence electrons of scandium are three.
The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating electrons are called cations.
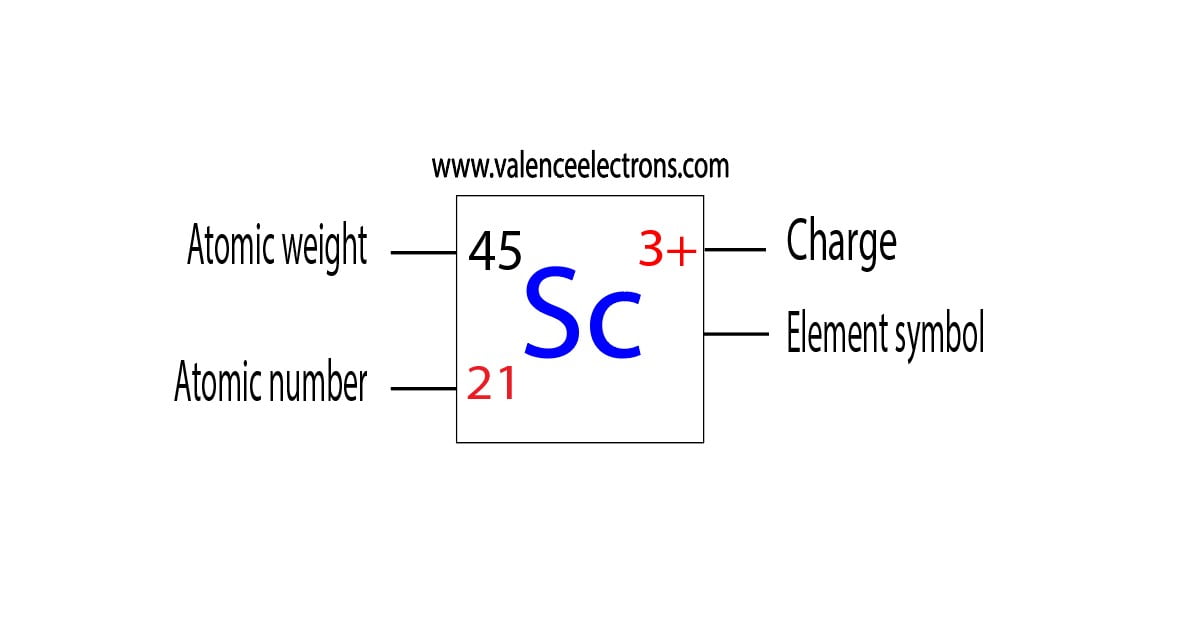
Scandium donates the electron of the last shell to form bonds and turns into a scandium ion(Sc3+). That is, scandium is a cation element.
Sc – 3e– → Sc3+
The electron configuration of scandium ion(Sc3+) is 1s2 2s2 2p6 3s2 3p6. This electron configuration shows that scandium ion(Sc3+) has acquired the electron configuration of argon and it achieves an octave full stable electron configuration.
Compound formation of scandium
Scandium participates in the formation of bonds through its valence electrons. We know that the valence electrons in scandium are three.
This valence electron participates in the formation of bonds with atoms of other elements. The electron configuration of oxygen shows that the valence electrons of oxygen are six.
The scandium atom donates its valence electrons to the oxygen atom and the oxygen atom receives those electrons. As a result, oxygen acquires the electron configuration of neon, and scandium atoms acquire the electron configuration of argon.
Scandium oxide(Sc2O3) is formed by the exchange of electrons between two atoms of scandium and three atoms of oxygen. Scandium oxide(Sc2O3) is an ionic compound.
FAQs
What is the symbol for scandium?
Ans: The symbol for scandium is ‘Sc’.
How many electrons does scandium have?
Ans: 21 electrons.
How do you write the full electron configuration for scandium?
Ans: 1s2 2s2 2p6 3s2 3p6 3d1 4s2.
How many valence electrons does scandium have?
Ans: Three valence electrons.
What is the valency of scandium?
Ans: The valency of scandium is 3.