How to Find the Valence Electrons for Carbon Disulfide?
The total number of electrons in the last orbit of an element is called the valence electrons of that element. Two or more elements combine to form compounds.
The valence electrons of a compound are the sum of the total valence electrons of each element in that compound. Carbon disulfide consists of two basic atoms. One is a carbon atom and the other is a sulfur atom.
The valence electrons of carbon disulfide are the sum of the total valence electrons of carbon and sulfur in the compound CS2. The carbon disulfide compound has a total of sixteen electrons in the last orbits of carbon and sulfur.
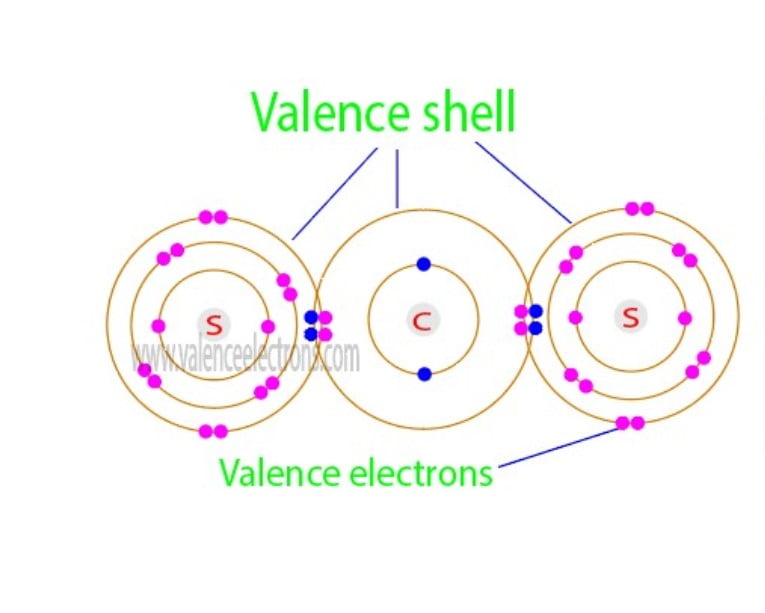
Hence, the total number of valence electrons in carbon disulfide is sixteen. To determine the valence electrons of a compound, the number of valence electrons in each element of the compound must be known.
This article discusses in detail how to determine the valence electrons of carbon disulfide very easily. Hopefully, after reading this article you will know more about this topic.
How to easily determine the number of valence electrons of CS2?
The symbol form of carbon disulfide is CS2. To determine the valence electrons of CS2, it is first necessary to know the valence electrons of the sulfur and carbon atoms.
To determine the valence electrons of carbon disulfide we have to follow two steps. It is shown below:
Step 1: Determine the valence electrons of carbon and sulfur atoms
The atomic number of carbon is 6. So its total number of electrons is six. The electron configuration of carbon shows that it has four electrons in its last orbit.
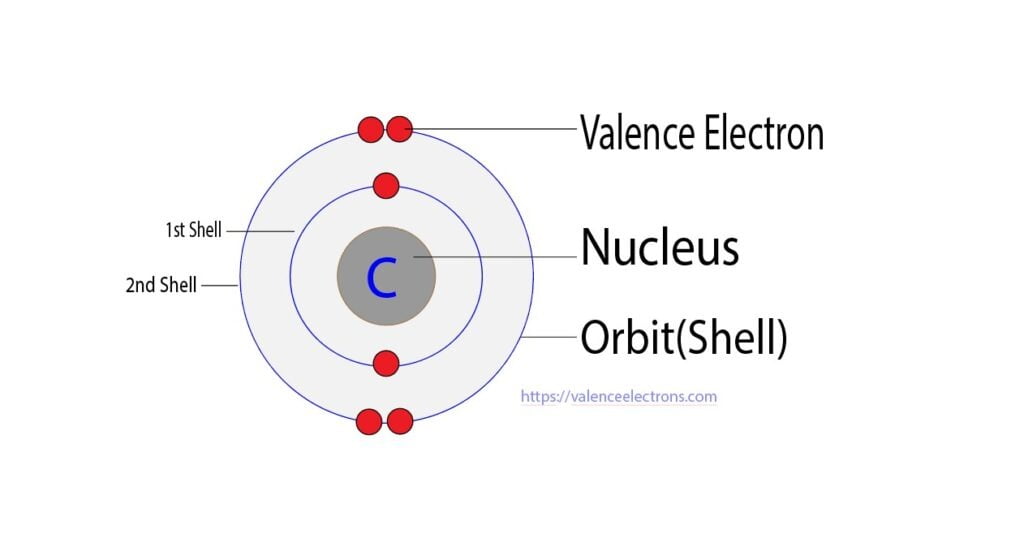
We already know that the electrons in the last orbit of an element are the valence electrons of that element. Therefore, the valence electrons of carbon are four.
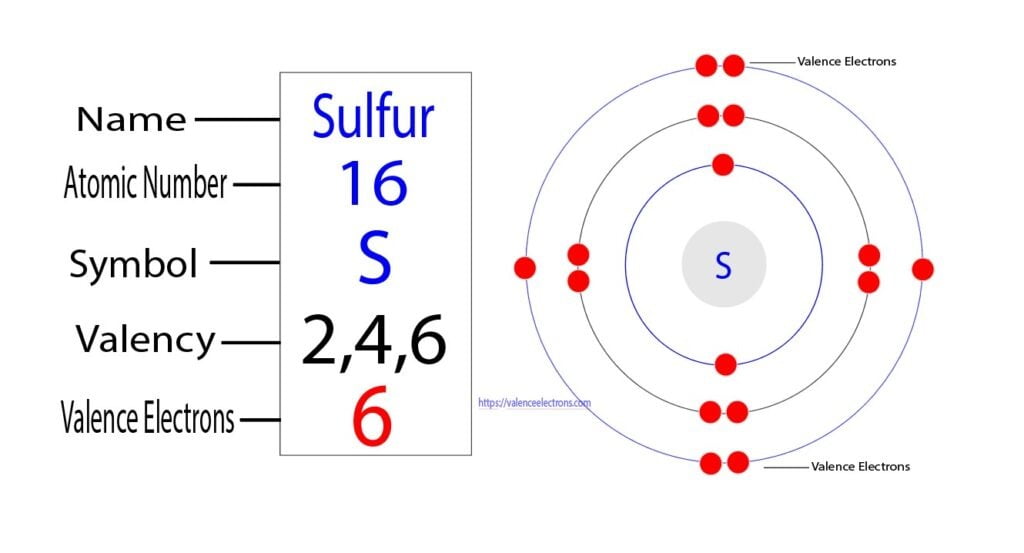
On the other hand, the atomic number of sulfur is 16. So its total number of electrons is sixteen. The electron configuration of sulfur shows that it has six electrons in its last orbit. Therefore, the valence electrons of sulfur are six.
Step 2: Determine the total number of valence electrons in the carbon disulfide compound
Carbon disulfide is a compound. It is composed of one carbon atom and two sulfur atoms.
Therefore, adding the valence electrons of one carbon atom and the valence electrons of two sulfur atoms can easily determine the valence electrons of carbon disulfide.
Mathematical Analysis:
CS2
= 4 + (6×2)
= 16
| Carbon (C) | Sulfur (S) | Carbon disulfide (CS2) |
| 4 | 6 | 16 |
From the above mathematical analysis, we can see that the total number of electrons in the outermost orbit of carbon disulfide is sixteen. Therefore, the valence electrons of carbon disulfide are sixteen.
Why do you need to know the valence electrons of an element?
In addition to knowing the number of electrons of an element, it is also necessary to know the number of valence electrons of that element. The number of valence electrons of an element carries important properties of that element.
Many important properties of elements and compounds can be determined by valence electrons. Some important features are mentioned below:
- Determining the element’s position in the periodic table
- Determining the number of groups of elements in the periodic table
- Determining Valency
- Lewis dot formation
- Determination of the molecular structure of the compound
- Determination of bond pairs and non-pair electrons of compounds
Valence electrons are related to the characteristics of a compound. So we need to know the valence electrons of all the elements to determine the properties of compounds.