How to Find the Valence Electrons for Strontium (Sr)?
The 38th element of the periodic table is strontium. The element of group-2 is strontium and its symbol is ‘Sr’. Strontium is an alkaline earth metal. Strontium forms bonds through its valence electrons.
What are the valence electrons of strontium?
The 4th element in group-2 is strontium. All the elements of group-2 are called alkaline earth metals. Therefore, strontium is an alkaline earth metal. The valence electron is the total number of electrons in the last orbit.
The total number of electrons in the last shell after the electron configuration of strontium is called the valence electrons of strontium. The valence electrons determine the properties of the element and participate in the formation of bonds.
How do you calculate the number of valence electrons in a strontium atom?
The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electrons without electron configuration.
Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all the elements.
However, valence electrons can be easily identified by arranging electrons according to the Bohr principle. Now we will learn how to determine the valence electrons of strontium.
Step-1: Determining the total number of electrons in strontium
1st we need to know the total number of electrons in the strontium atom. To know the number of electrons, you need to know the number of protons in strontium. And to know the number of protons, you need to know the atomic number of the strontium element.
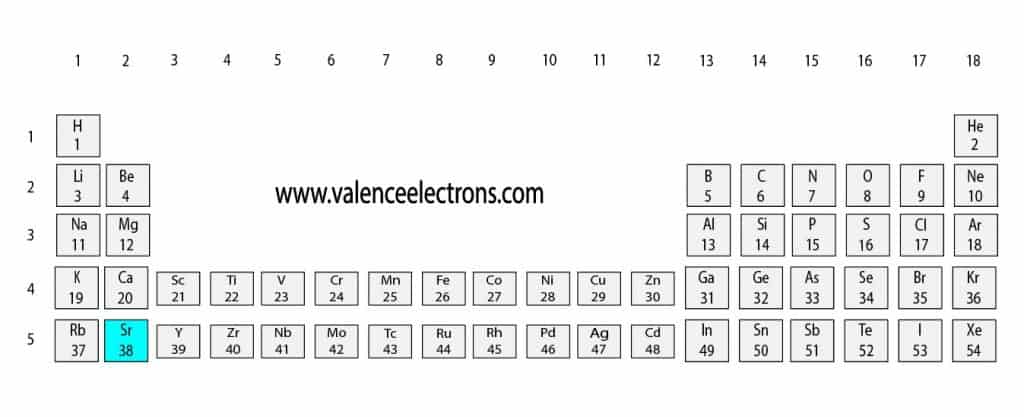
To know the atomic number we need to take the help of a periodic table. It is necessary to know the atomic number of strontium elements from the periodic table. The atomic number is the number of protons. And electrons equal to protons are located outside the nucleus.
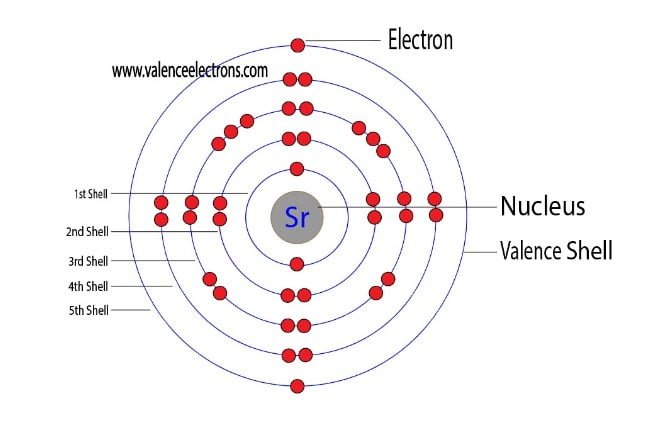
That is, we can finally say that there are electrons equal to the atomic number in the strontium atom. From the periodic table, we see that the atomic number of strontium is 38. That is, the strontium atom has a total of thirty-eight electrons.
Step-2: Need to do electron configuration of strontium
Step-2 is very important. In this step, the electrons of strontium have to be arranged. We know that strontium atoms have a total of thirty-eight electrons.
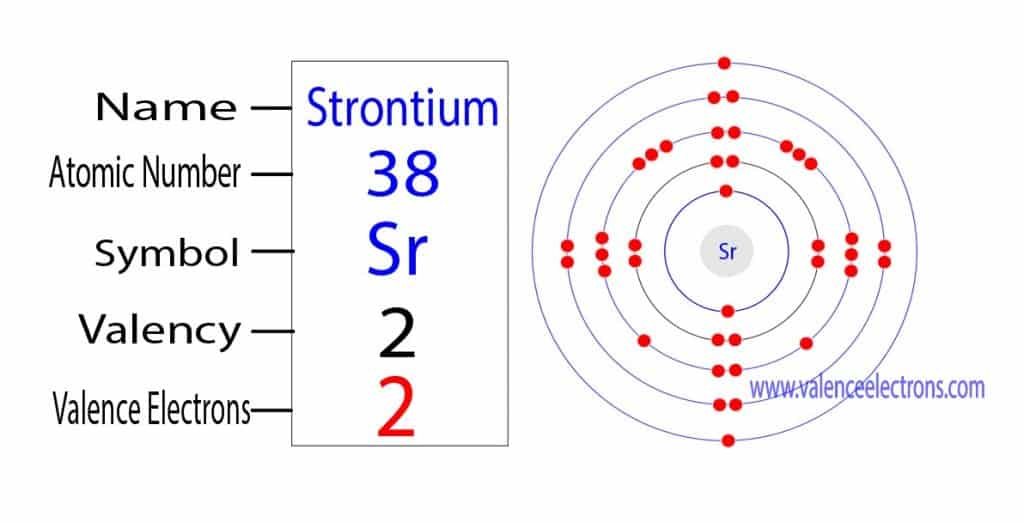
That is, the first shell of strontium has two electrons, the second shell has eight electrons, 3rd shell has eighteen electrons, 4th shell has eight electrons and the 5th shell has two electrons. The number of electrons per shell of strontium is 2, 8, 18, 8, 2.
Step-3: Determine the valence shell and calculate the total electrons
The third step is to diagnose the valence shell. The last shell after the electron configuration is called the valence shell. The total number of electrons in a valence shell is called valence electrons.
The electron configuration shows that the last shell of strontium has two electrons. Therefore, the valence electrons of strontium are two.
Video for Find the Valence Electrons for Strontium (Sr)
What is the valency of strontium?
The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an element is the valency of that element.
During compound formation, the strontium atom donates two electrons from its valence shell to another atom. Therefore, the valency of strontium is 2.
How many valence electrons does strontium ion(Sr2+) have?
The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating electrons are called cations.
Strontium atom donates two electrons of the last shell to form bonds and turns into a strontium ion(Sr2+). That is, strontium is a cation element.
Sr – 2e– → Sr2+
The electron configuration of strontium ion is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. This electron configuration shows that the strontium ion has four shells and the last shell has eight electrons(4s2 4p6) and the strontium ion(Sr2+) has acquired the electron configuration of krypton.
Since the last shell of a strontium ion has eight electrons, the valence electrons of the strontium ion(Sr2+) are eight.