How to Find the Valence Electrons for Manganese (Mn)?
The 25th element in the periodic table is manganese. The element of group-7 is manganese and its symbol is ‘Mn’. Manganese is a transition element. Therefore, the valence electrons of manganese are determined differently.
This article discusses in detail how to easily calculate the number of valence electrons in manganese. Hopefully, after reading this article you will know in detail about this.
What are the valence electrons of manganese?
The 1st element in group-7 is manganese. The elements in groups 3-12 are called transition elements. The valence electrons are the total number of electrons in the last orbit.
But in the case of transition elements, the valence electrons remain in the inner shell(orbit).
Because the electron configuration of manganese shows that the last electrons enter the d-orbital. The valence electrons determine the properties of the element and participate in the formation of bonds.
How do you calculate the number of valence electrons in a manganese atom?
The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration.
Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all elements.
The valence electrons of the transition element cannot be determined according to Bohr’s atomic model. Because the valence electrons of the transition elements are located in the inner shell.
However, the valence electron of the transition element can be easily determined according to the Aufbau principle. Now we will learn how to determine the valence electron of manganese.
Step-1: Determining the total number of electrons in manganese
1st we need to know the total number of electrons in the manganese atom. To know the number of electrons, you need to know the number of protons in manganese.
And to know the number of protons, you need to know the atomic number of the manganese element.
To know the atomic number we need to take the help of a periodic table. It is necessary to know the atomic number of manganese elements from the periodic table.
The atomic number is the number of protons. And electrons equal to protons are located outside the nucleus.
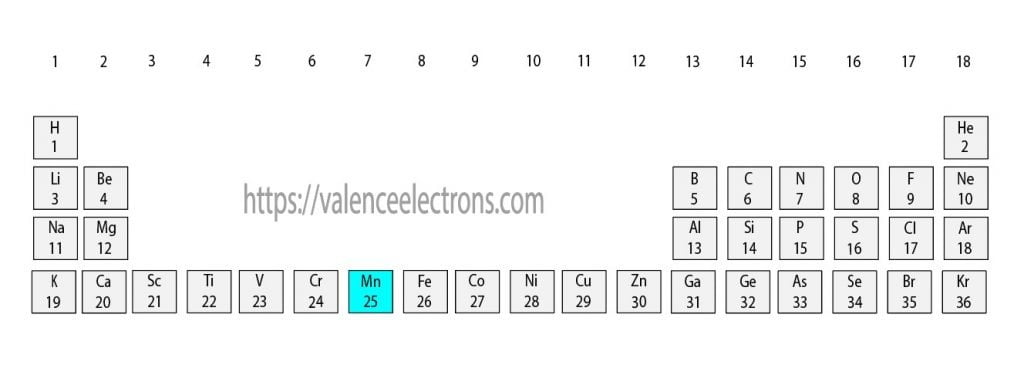
That is, we can finally say that there are electrons equal to the atomic number in the manganese(Mn) atom.
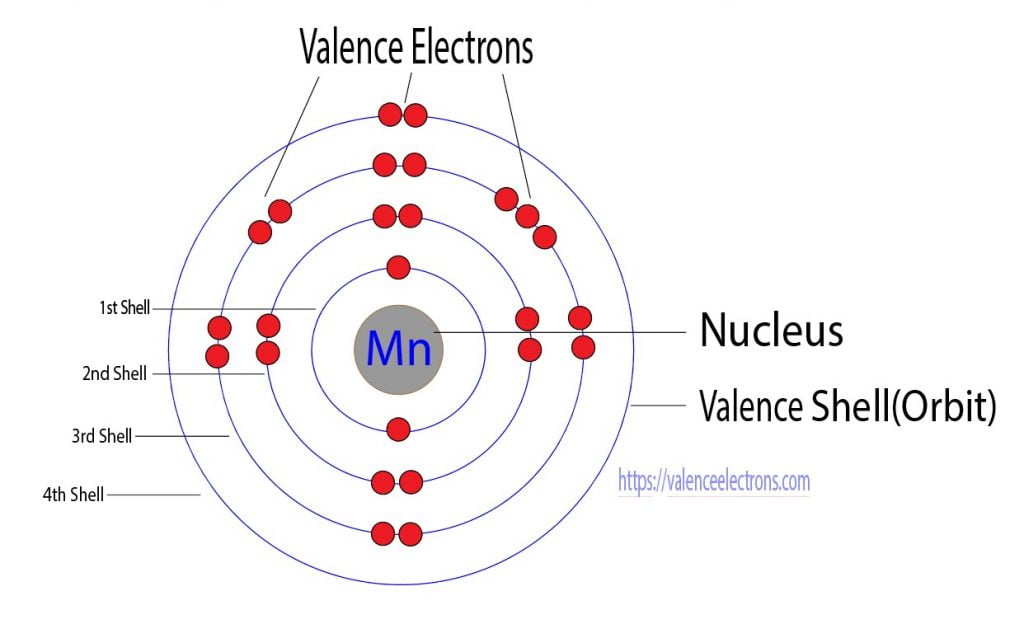
From the periodic table, we see that the atomic number of manganese is 25. That is, the manganese atom has a total of twenty-five electrons.
Step-2: Need to do electron configuration of manganese
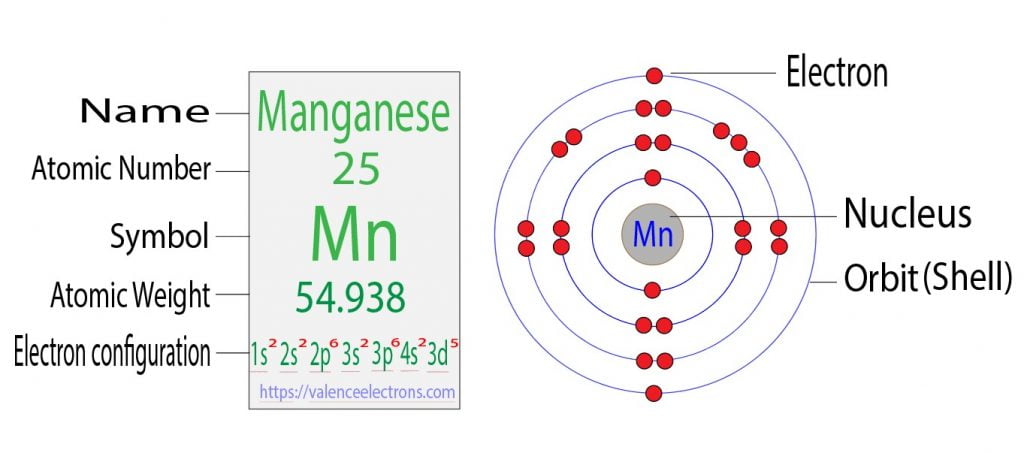
Step 2 is very important. In this step, the electrons of manganese have to be arranged. We know that manganese atoms have a total of twenty-five electrons.
The electron configuration shows that the first shell of manganese has two electrons, the second shell has eight electrons, the 3rd shell has thirteen electrons and the 4th shell has two electrons.
Step-3: Determine the valence shell and calculate the total electrons
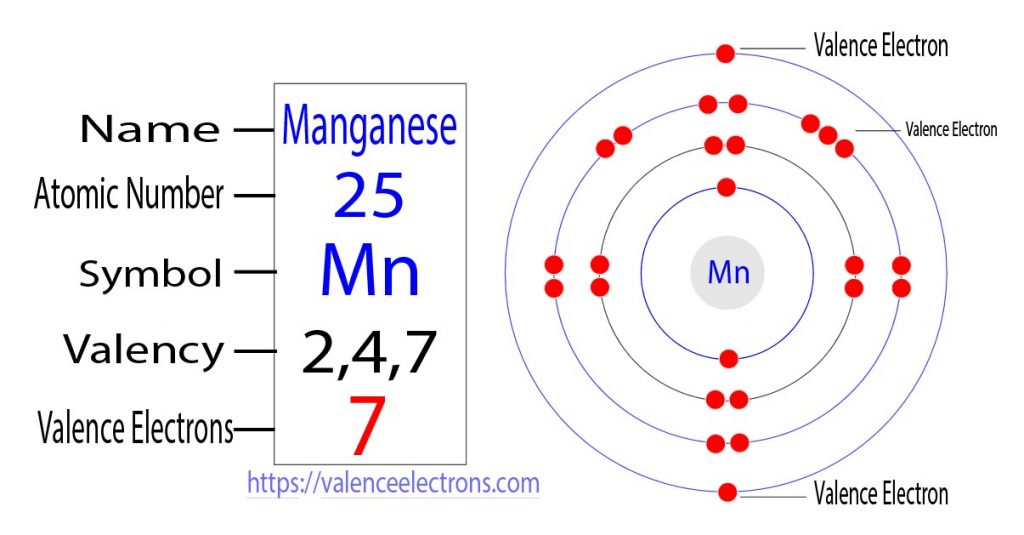
The third step is to diagnose the valence shell(orbit). The last shell after the electron configuration is called the valence shell. The total number of electrons in a valence shell is called valence electrons.
But the valence electrons of the transition elements are located in the inner orbit. For the transition element, the valence electrons have to be determined by adding the total electrons of the d-orbital to the electrons in the last orbit of the atom.
The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons. Therefore, the valence electrons of manganese are seven.
Video for Find the Valence Electrons for Manganese (Mn)
What is the valency of manganese?
The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some rules for diagnosing valency.
The number of electrons in an unpaired state in the last shell after the electron configuration of an atom is called the valency of that element.
The oxidation states of manganese are +2, +3, +4, +6, and +7. The oxidation state of manganese +2 has been used in the manganese(II) oxide(MnO) bond. The valency of manganese in this compound is 2.
On the other hand, the oxidation state of manganese +7 has been used in permanganate ion(MnO4)1-. The valency of manganese in this compound is 7. The valency and oxidation states depend on the bond formation.
How many valence electrons does manganese ion(Mn2+, Mn3+, Mn4+) have?
The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating electrons are called cation.
There are three types of manganese ion. The manganese atom exhibits Mn2+, Mn3+, and Mn4+ ion. The manganese atom donates two electrons from the last shell to form the manganese ion(Mn2+).
Mn – 2e– → Mn2+
Here, the electron configuration of manganese ion(Mn2+) is 1s2 2s2 2p6 3s2 3p6 3d5.
This electron configuration shows that the manganese ion has three shells and the last shell has thirteen electrons.
For this, the manganese ion(Mn2+) has a total of thirteen valence electrons. The manganese atom donates two electrons in the 4s orbital and an electron in the 3d orbital to convert to a manganese ion(Mn3+).
Mn – 3e– → Mn3+
On the other hand, this electron configuration of manganese ion(Mn3+) is 1s2 2s2 2p6 3s2 3p6 3d4. Here, the manganese ion has three shells and that shell has twelve electrons.
Therefore the valence electrons of the manganese ion(Mn3+) are twelve. Again, the manganese atom donates two electrons in the 4s orbital and two electrons in the 3d orbital to convert to a manganese ion(Mn4+).
Mn – 4e– → Mn4+
This electron configuration of manganese ion(Mn4+) is 1s2 2s2 2p6 3s2 3p6 3d3.
Here, the manganese ion has three shells and the last shell has eleven electrons. In this case, the valence electrons of manganese ion(Mn4+) are eleven.