How to Find the Valence Electrons for PCl3?
The total number of electrons in the last orbit of an element is called the valence electrons of that element. Two or more elements combine to form compounds.
The valence electrons of a compound are the sum of the total valence electrons of each element in that compound. Phosphorus trichloride consists of two atoms. One is a phosphorus atom and the other is a chlorine atom.
The valence electrons of phosphorus trichloride are the sum of the total valence electrons of phosphorus and chlorine in the compound PCl3.
The phosphorus trichloride compound has a total of twenty-six electrons in the last orbits of phosphorus and chlorine.
Hence, the total number of valence electrons in phosphorus trichloride is twenty-six. To determine the valence electrons of a compound, the number of valence electrons in each element of the compound must be known.
This article discusses in detail how to determine the valence electrons of phosphorus trichloride very easily. Hopefully, after reading this article you will know more about this topic.
How to easily determine the number of valence electrons of PCl3?
The symbol form of phosphorus trichloride is PCl3. To determine the valence electrons of PCl3, it is first necessary to know the valence electrons of the chlorine and phosphorus atoms.
To determine the valence electrons of phosphorus trichloride we have to follow two steps. It is shown below:
Step 1: Determine the valence electrons of phosphorus and chlorine atoms
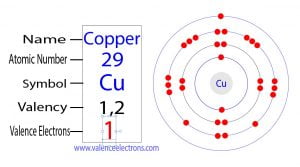
The atomic number of phosphorus is 15. So its total number of electrons is fifteen. The electron configuration of phosphorus shows that it has five electrons in its last orbit.
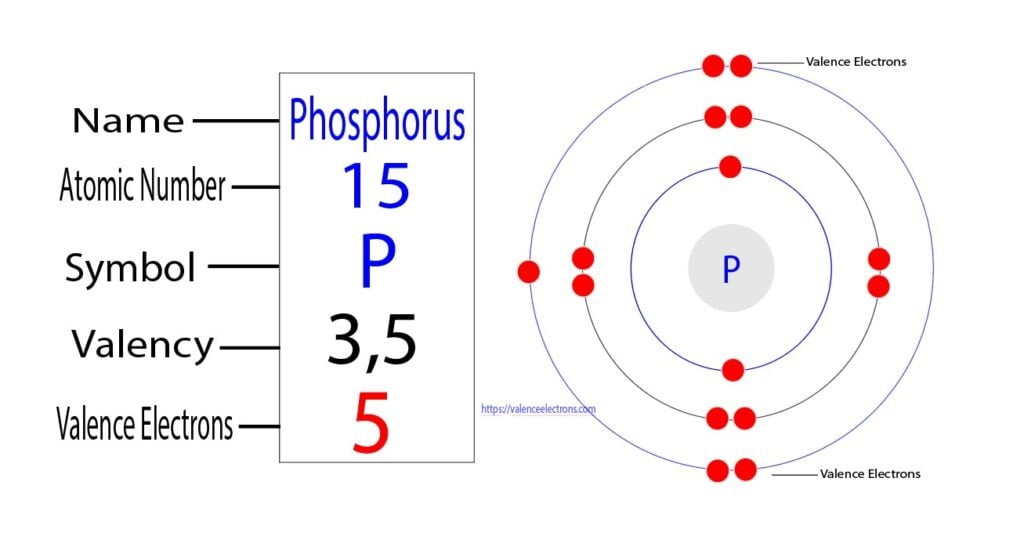
We already know that the electrons in the last orbit of an element are the valence electrons of that element. Therefore, the valence electrons of phosphorus are five.
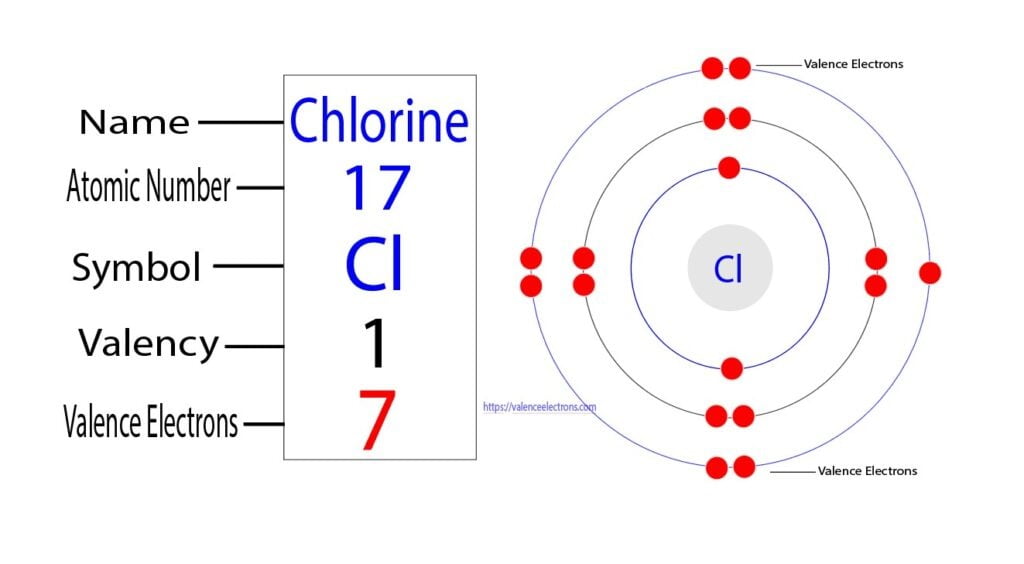
On the other hand, the atomic number of chlorine is 17. So its total number of electrons is seventeen. The electron configuration of chlorine shows that it has seven electrons in its last orbit. Therefore, the valence electrons of chlorine are seven.
Step 2: Determine the total number of valence electrons in the phosphorus trichloride compound
Phosphorus trichloride is a compound. It is composed of one phosphorus atom and three chlorine atoms.
Therefore, adding the valence electrons of one phosphorus atom and the valence electrons of three chlorine atoms can easily determine the valence electrons of phosphorus trichloride.
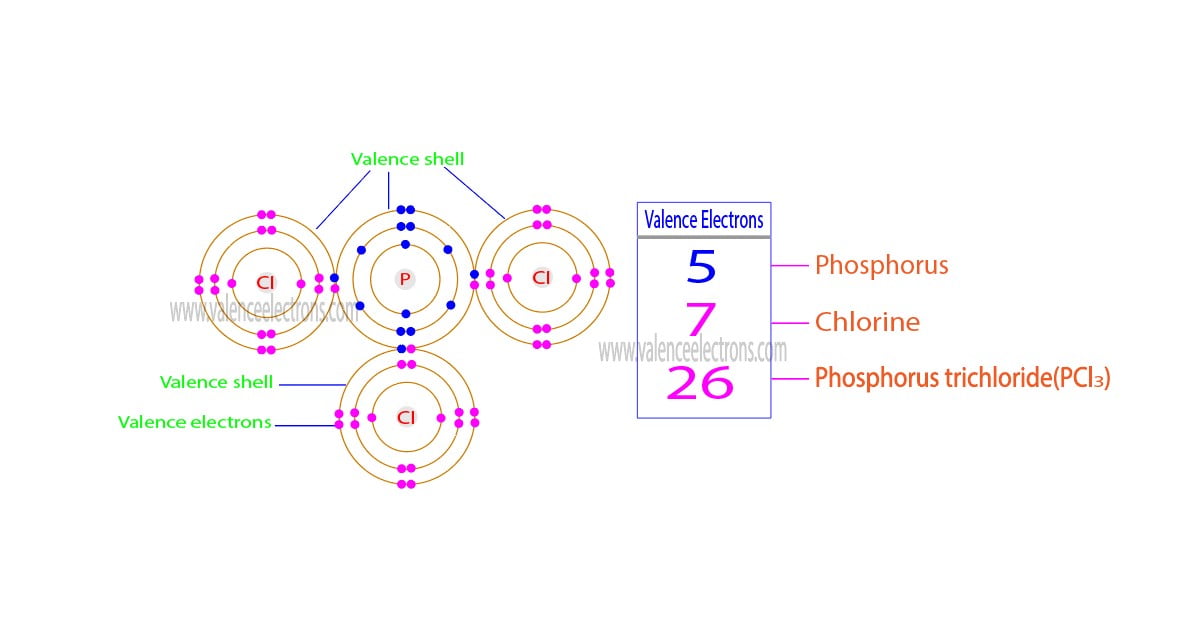
Mathematical Analysis:
PCl3
= 5 + (7×3)
= 26
| Phosphorus (P) | Chlorine (Cl) | Phosphorus trichloride (PCl3) |
| 5 | 7 | 26 |
From the above mathematical analysis, we can see that the total number of electrons in the outermost orbit of phosphorus trichloride is twenty-six. Therefore, the valence electrons of phosphorus trichloride are twenty-six.
Why do you need to know the valence electrons of an element?
In addition to knowing the number of electrons of an element, it is also necessary to know the number of valence electrons of that element. The number of valence electrons of an element carries important properties of that element.
Many important properties of elements and compounds can be determined by valence electrons. Some important features are mentioned below:
- Determining the element’s position in the periodic table
- Determining the number of groups of elements in the periodic table
- Determining Valency
- Lewis dot formation
- Determination of the molecular structure of the compound
- Determination of bond pairs and non-pair electrons of compounds
Valence electrons are related to the characteristics of a compound. So we need to know the valence electrons of all the elements to determine the properties of compounds.