How to Write the Orbital Diagram for Silver (Ag)?
The silver orbital diagram is a graphical representation of the electron configuration of the silver atom.
This diagram shows how the electrons in the silver atom are arranged in different orbitals.
Orbital is the region of space around the nucleus of an atom where electrons are found.
To create an orbital diagram of silver, you first need to know the atomic orbitals and the orbital notation for the silver atom, and also you need to know Hund’s principle.
What is orbital?
The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit (shell). Again, atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital.
The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’.
| Orbit Number | Value of ‘l’ | Number of subshells | Number of orbital | Subshell name | Electrons holding capacity | Electron Configuration |
| 1 | 0 | 1 | 1 | 1s | 2 | 1s2 |
| 2 | 0 1 | 2 | 1 3 | 2s 2p | 2 6 | 2s2 2p6 |
| 3 | 0 1 2 | 3 | 1 3 5 | 3s 3p 3d | 2 6 10 | 3s2 3p6 3d10 |
| 4 | 0 1 2 3 | 4 | 1 3 5 7 | 4s 4p 4d 4f | 2 6 10 14 | 4s2 4p6 4d10 4f14 |
The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, and f.
The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell, and seven in the f-subshell. Each orbital can have a maximum of two electrons.
| Sub-shell name | Name source | Value of ‘l’ | Value of ‘m’ (0 to ± l) | Number of orbital (2l+1) | Electrons holding capacity 2(2l+1) |
| s | Sharp | 0 | 0 | 1 | 2 |
| p | Principal | 1 | −1, 0, +1 | 3 | 6 |
| d | Diffuse | 2 | −2, −1, 0, +1, +2 | 5 | 10 |
| f | Fundamental | 3 | −3, −2, −1, 0, +1, +2, +3 | 7 | 14 |
The sub-energy level ‘s’ can hold a maximum of two electrons, ‘p’ can hold a maximum of six electrons, ‘d’ can hold a maximum of ten electrons, and ‘f’ can hold a maximum of fourteen electrons.
What is the orbital notation for silver?
The silver orbital notation is a shorthand system designed to represent the exact positions of the electrons in the silver atom. This is similar to electron configuration, but numbers are used instead of boxes to represent the positions of the electrons.
This orbital notation system always follows the Aufbau principle. The Aufbau principle is that the electrons present in the atom will first complete the lowest energy orbital and then gradually continue to complete the higher energy orbital.
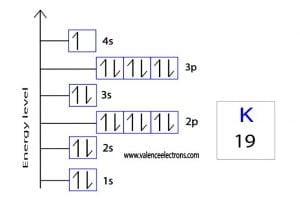
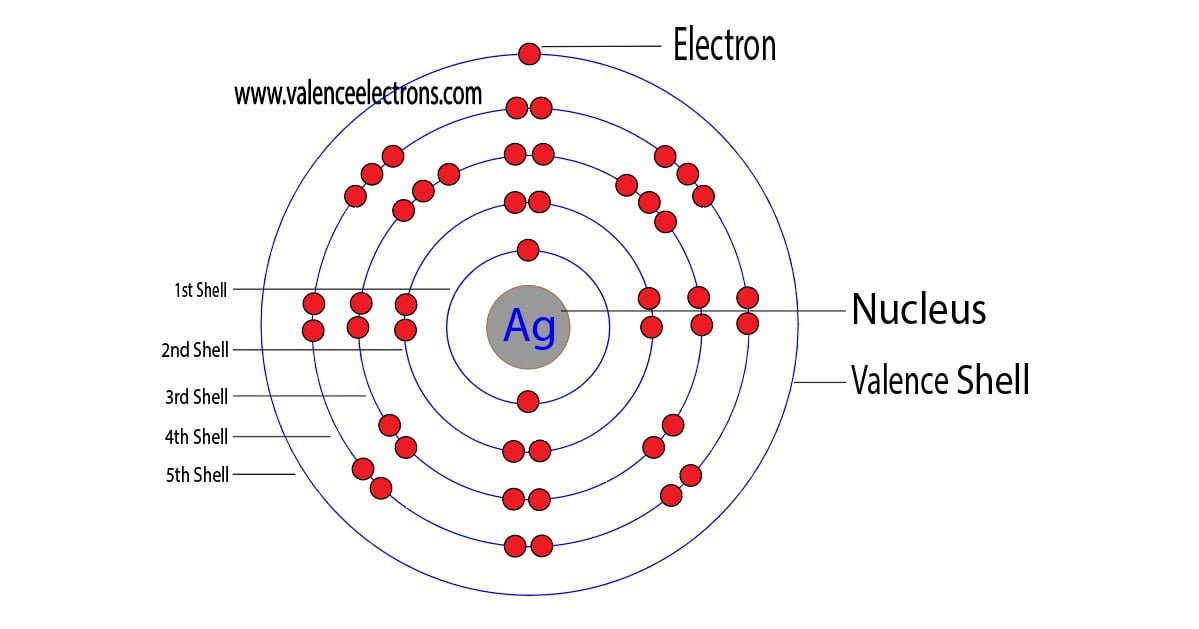
The atomic number of silver is 47, which means it has 47 electrons. Now it is possible to find the orbital notation of silver very easily through electron configuration. That is, the orbital notation of silver is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10.
What is Hund’s principle?
Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when multiple orbitals of the same energy level are available.
Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way.
For example, we already know that the p-subshell has three orbitals. The orbitals are px, py, and pz and each orbital can have a maximum of two electrons. When electrons want to enter the p-subshell, then the first electron will enter the px orbital in the clockwise direction.
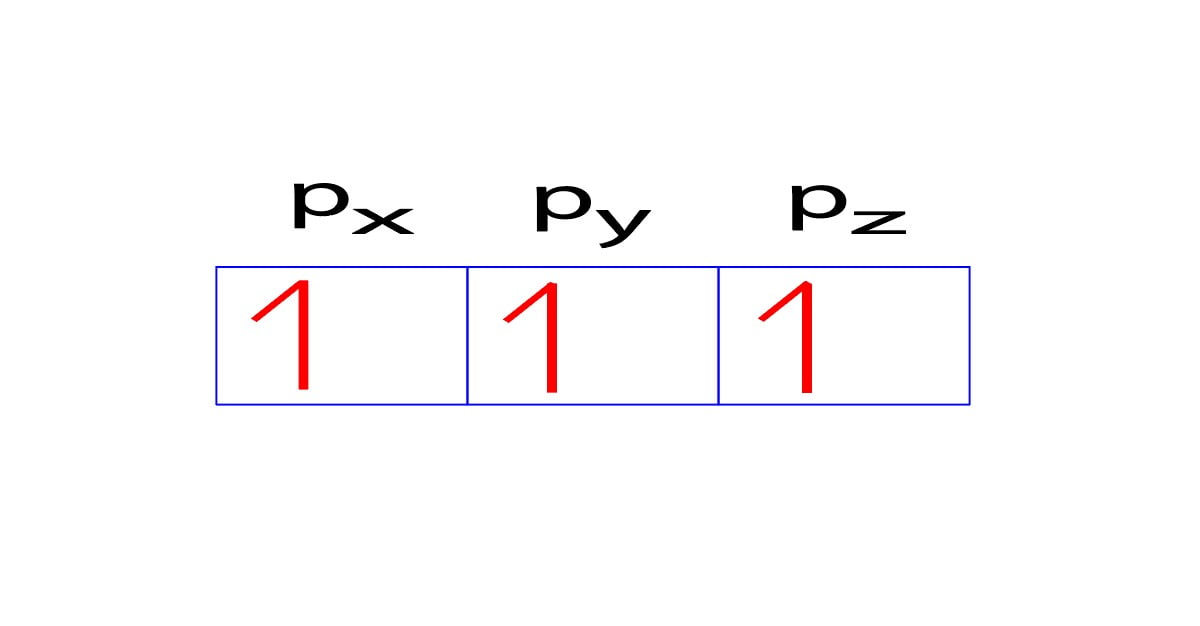
The second electron will also enter the py orbital in the clockwise direction and the third electron will also enter the pz orbital in the clockwise direction.
Now, when the fourth electron wants to enter the p-subshell, then it will enter the px orbital in the anti-clockwise direction.
The fifth electron will also enter the py orbital in the anti-clockwise direction and the sixth electron will also enter the pz orbital in the anti-clockwise direction.
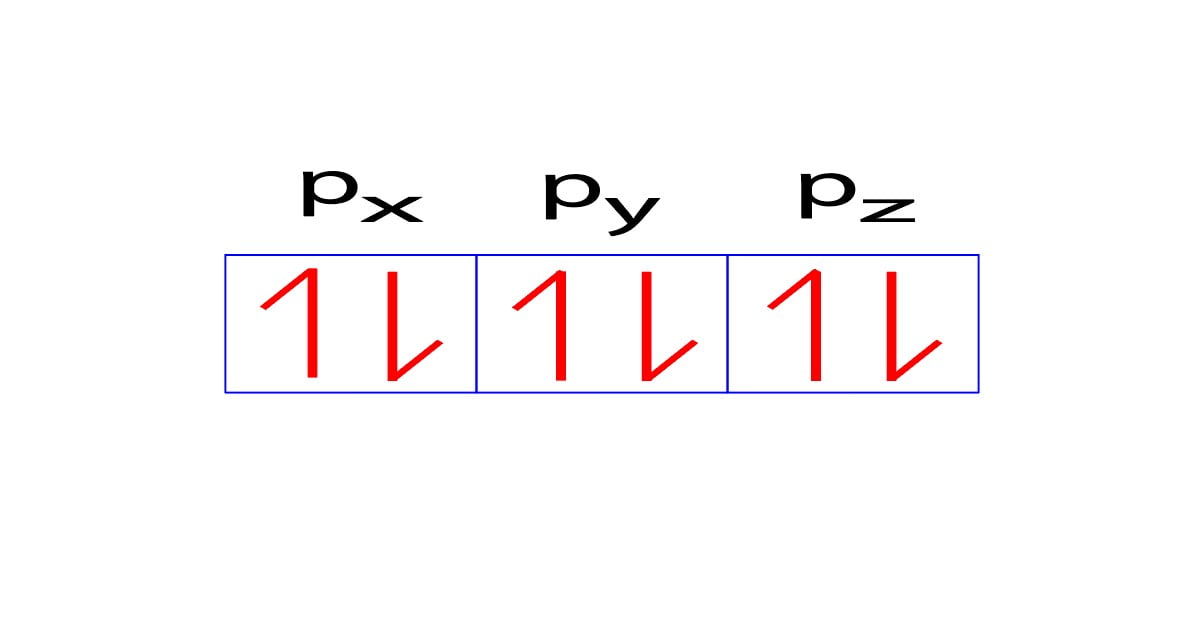
Here, the clockwise direction is expressed by the upper sign (↑) and the anti-clockwise direction by the lower sign (↓). Basically, these two symbols (↑↓) indicate the direction of electron spin.
How to write the orbital diagram for silver?
Orbital diagrams are usually represented by boxes. Each box represents an orbital and the arrows within the box represent the position of the electron. The boxes are arranged in order of energy of the orbitals.
The lowest energy orbitals are closest to the nucleus and the higher energy orbitals are progressively further away from the nucleus in order of their energy levels. To write the orbital diagram of silver, you have to write the orbital notation of silver. Which has been discussed in detail above.
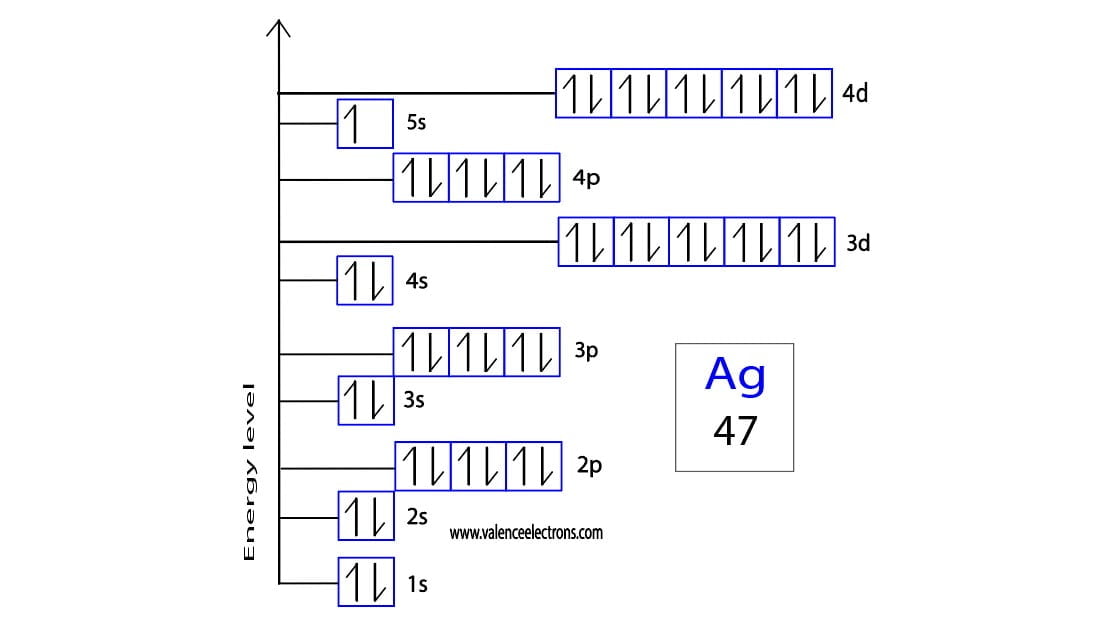
1s is the closest and lowest energy orbital to the nucleus. Therefore, the electrons will first enter the 1s orbital.
According to Hund’s principle, the first electron will enter 1s orbital in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
The 1s orbital is now filled with two electrons. The next two electrons will enter the 2s orbital just like the 1s orbital. The next three electrons will enter the 2p orbital in the clockwise direction and the next three electrons will enter the 2p orbital in the anti-clockwise direction.
The 2p orbital is now full. Then the next two electrons will enter the 3s orbital just like the 1s orbital. Then the next three electrons will enter the 3p orbital in the clockwise direction and the next three electrons will enter the 3p orbital in the anti-clockwise direction.
The 3p orbital is now full. So, the next two electrons will enter the 4s orbital just like the 1s orbital. The 4s orbital is now full. Therefore, the next five electrons will enter the 3d orbital in the clockwise direction and the next five electrons will enter the 3d orbital in the anti-clockwise direction.
The 3d orbital is now full. So, the next six electrons will enter the 4p orbital just like the 3p orbital. The 4p orbital is now full of electrons. So, an electron will enter the 5s orbital in the clockwise direction.
Then the next five electrons enter the 4d orbital in the clockwise direction and the remaining five electrons will enter the 4d orbital in the anti-clockwise direction. This is clearly shown in the figure of the orbital diagram of silver.