How Many Electrons are in the n=3 Shell of a Vanadium Atom?
Understanding the fundamental structure of an atom is crucial in the field of chemistry. It provides valuable insights into an element’s chemical properties and reactivity.
This article aims to answer a specific question: “How many electrons are in the n = 3 shell of a vanadium atom?”
To address this query, I will delve into atomic structure, quantum numbers, the element vanadium, and the rules governing electron distribution in electron shells.
Atomic Structure Basics
At the heart of this discussion is the atomic model. Atoms consist of three fundamental particles: protons, neutrons, and electrons. Electrons, which are negatively charged, orbit the nucleus of an atom.
Quantum Numbers
The behavior of electrons is described by quantum numbers, which include the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (m), and spin quantum number (s).
For our exploration, the principal quantum number (n) is of particular importance, as it relates to electron shells.
Vanadium Atom
Vanadium is a chemical element with the atomic number 23, which indicates the presence of 23 protons in its nucleus. To determine the electron distribution in a vanadium atom, we need to consider its electron configuration.
Electron Distribution in the n = 3 Shell
Electron distribution in atoms follows specific rules. The Aufbau principle is a fundamental concept, stating that electrons fill the lowest energy orbitals first.
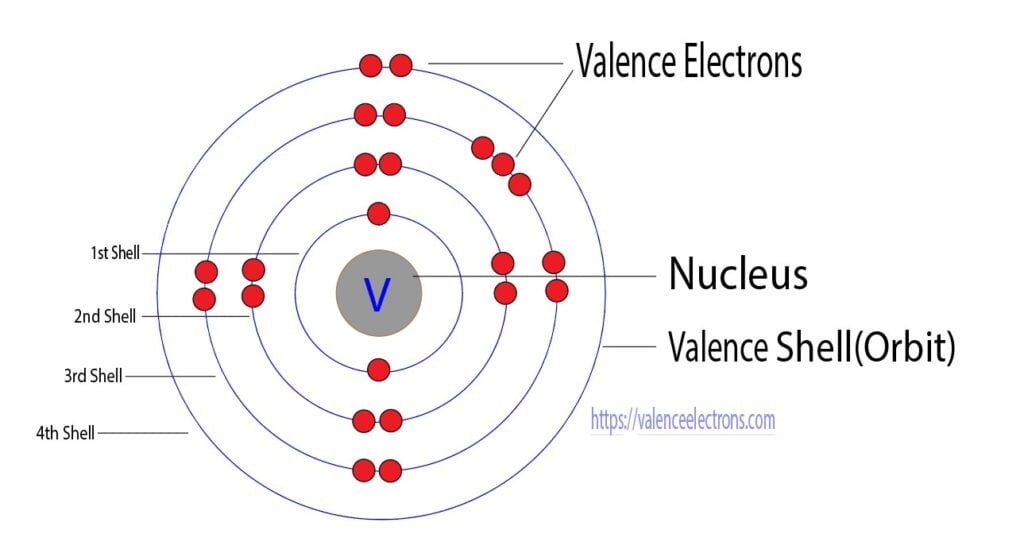
To determine the number of electrons in the n = 3 shell of a vanadium atom, we’ll follow these rules. Vanadium’s electron configuration is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d3.
To find the number of electrons in the n = 3 shell, we look at the electrons in the 3s, 3p, and 3d orbitals. There are a total of 2 + 6 + 3 = 11 electrons in the n = 3 shell of a vanadium atom.
Conclusion
In conclusion, there are eleven electrons in the n = 3 shell of a vanadium atom. Understanding electron distribution in atoms is essential for comprehending an element’s chemical behavior, reactivity, and role in chemical reactions.
This knowledge serves as a foundation for numerous aspects of chemistry, from chemical bonding to the creation of the periodic table.
